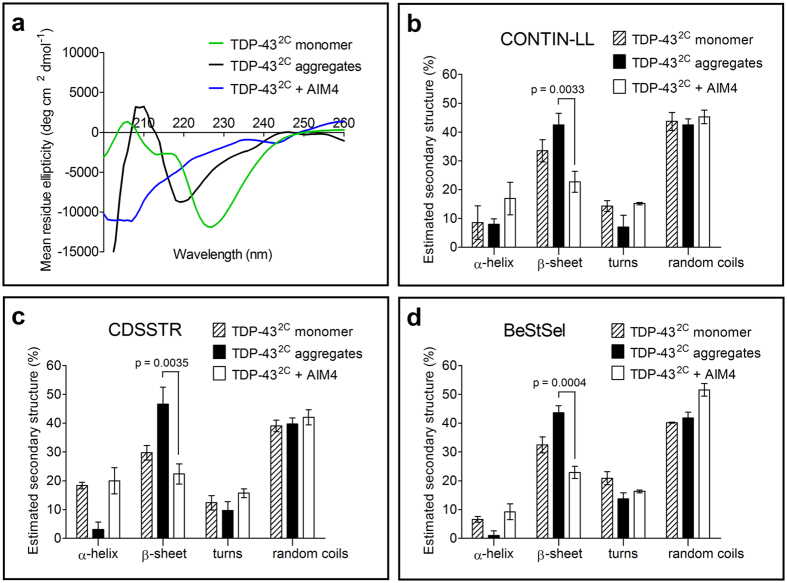
Figure 4. Effect of AIM4 on secondary structure of TDP-432C estimated by CD spectrometry.
(a) Far-UV circular dichroism (CD) spectra of TDP-432C in presence and absence of AIM4. Freshly prepared TDP-432C monomer in aggregation buffer, pre-formed TDP-432C aggregates and TDP-432C protein incubated similarly for aggregation with AIM4 were diluted in aggregation buffer and far-UV CD spectra were recorded and results have been expressed as mean residue ellipticity [ϴ]MRE. (b–d) Using the far-UV CD spectra from (a), the secondary structural contents were predicted by the online structure prediction tools: DichroWeb algorithms CONTIN & CDSSTR and server BeStSel. The relative contents of α-helix, β-sheet, turns and random coils predicted by these tools have been depicted as bar charts. The TDP-432C aggregation sample with AIM4 showed a decrease of ~20% in β-sheet content compared to those without AIM4. Error bars represent standard deviations of structural predictions obtained from the CD spectra from three samples (n = 3).