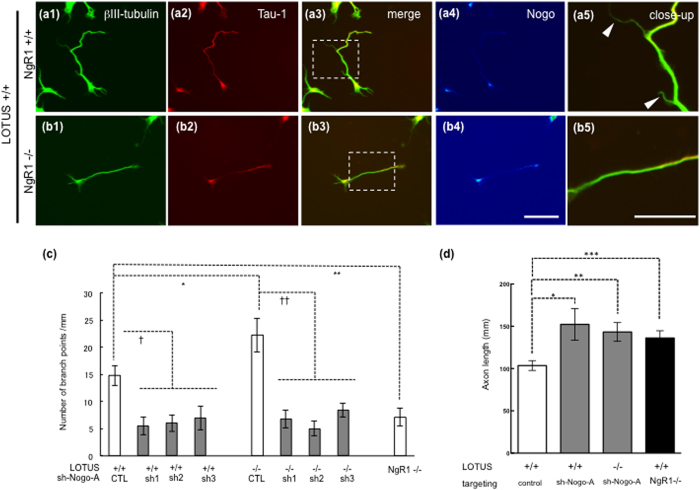
Figure 5. NgR1 mediates axonal collateral formation in cultured OB neurons.
(a1,b1) Immunostaining of βIII-tubulin, a neuronal marker. (a2,b2) Immunostaining of Tau-1, an axonal marker in the same cells of (1), (a3,b3) merged images of (1) and (2), (a4,b4) and immunostaining of Nogo-A in pseudo-color. (a5,b5) Dashed boxes in images of a3 and b3 correspond to images of a5 and b5 at higher magnification, respectively. Arrowheads indicate axonal collateral branches. Dissociated OB neurons from E14.5 wild type mice (NgR1+/+) (a) or ngr1-deficient mice (NgR1−/−) (b) were cultured for 7days. Branching points in ngr1-deficient (NgR1−/−) mice were decreased in comparison with the wild type. (c) Quantitative analysis of axonal branching points in cultured OB neurons. Significance, indicated by (*), was obtained by performing Student’s unpaired t-test. *is P = 0.0437 (LOTUS+/+ versus LOTUS−/− (n = 4)), ** is P = 0.0029 (NgR1+/+ versus NgR1−/− (n = 3)), and comparisons indicated by (†) were obtained by performing a one-way ANOVA test with all pairwise multiple comparisons test (Tukey’s test). † is P < 0.05. †† is P < 0.000001. Scale bars: 50 μm.(d) Quantitative analysis of axon lengths in cultured OB neurons. Axon lengths of Tau-1-positive neurites were measured using NIH ImageJ software. Nogo-A knockdown (sh-Nogo, using sh-1 in LOTUS+/+ and sh-3 in LOTUS−/−) resulted in the increase of axon length. Similarly, axon lengths in ngr1-deficient (NgR1−/−) mice were increased compared to those in the wild-type mice. Significance was obtained by performing one-way ANOVA with Holm-Sidak’s multiple comparison test. *P = 0.0192 (control versus sh-Nogo treatment (n = 17–34 axons)); **P = 0.0218 (wild type versus NgR1−/− (n = 4 littermates)); ***P = 0.0229 (wild type versus LOTUS−/− (n = 5 littermates)).