Fig. 1.
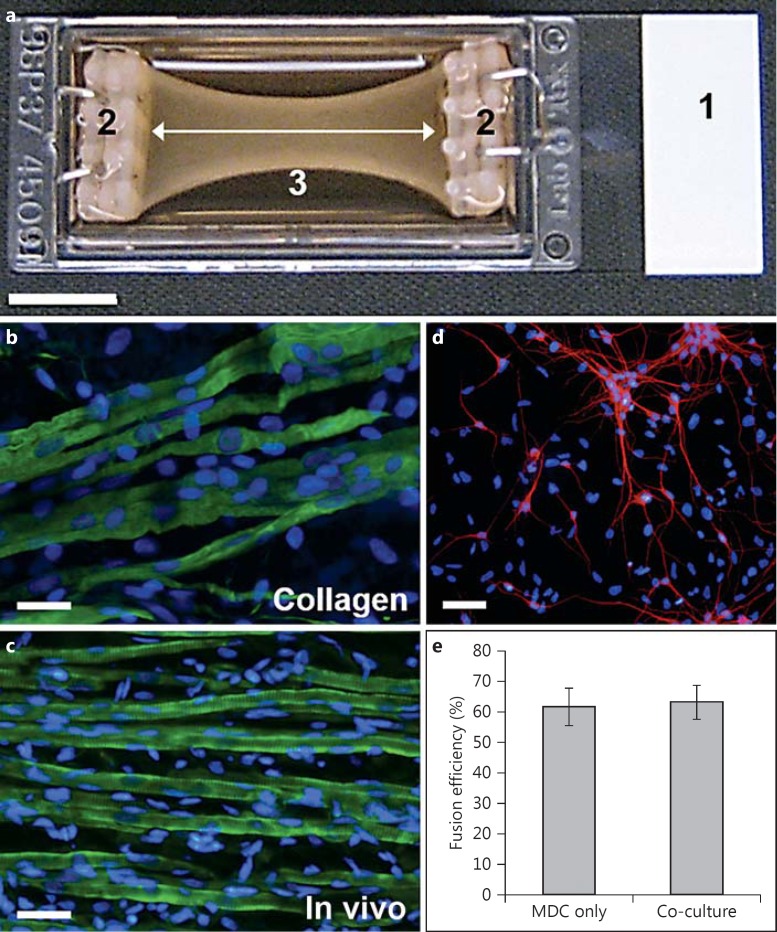
3D co-culture platform and cell population characterization. a A chamber slide (1) fitted with custom-built flotation bars at either end (2). A cell-laden collagen solution is allowed to gel between the flotation bars. The collagen gel is then detached from the edges of the chamber slide so that it floats in the culture medium. Cell-mediated matrix compaction leads to the generation of isometric strain between the flotation bars (white arrow). Compaction of the collagen gel over time produces the characteristic bowing illustrated (3). Scale bar = 10 mm. b Image of MDCs maintained in this culture platform for 2 weeks and stained for desmin (green) and nuclei (blue). Note the parallel alignment of the developing myotubes. Scale bar = 20 μm. c Image of a muscle tissue section taken from a P1 rat pup, cryosectioned, and stained for desmin (green) and nuclei (blue). Scale bar = 20 μm. d Image of ventral horn motor neurons maintained on glass coverslips for 7 days before being stained for MAP-2 (red) and nuclei (blue). Scale bar = 40 μm. e Comparison of fusion efficiency in MDC-only 3D constructs versus motor neuron-muscle co-cultures. p = 0.86.