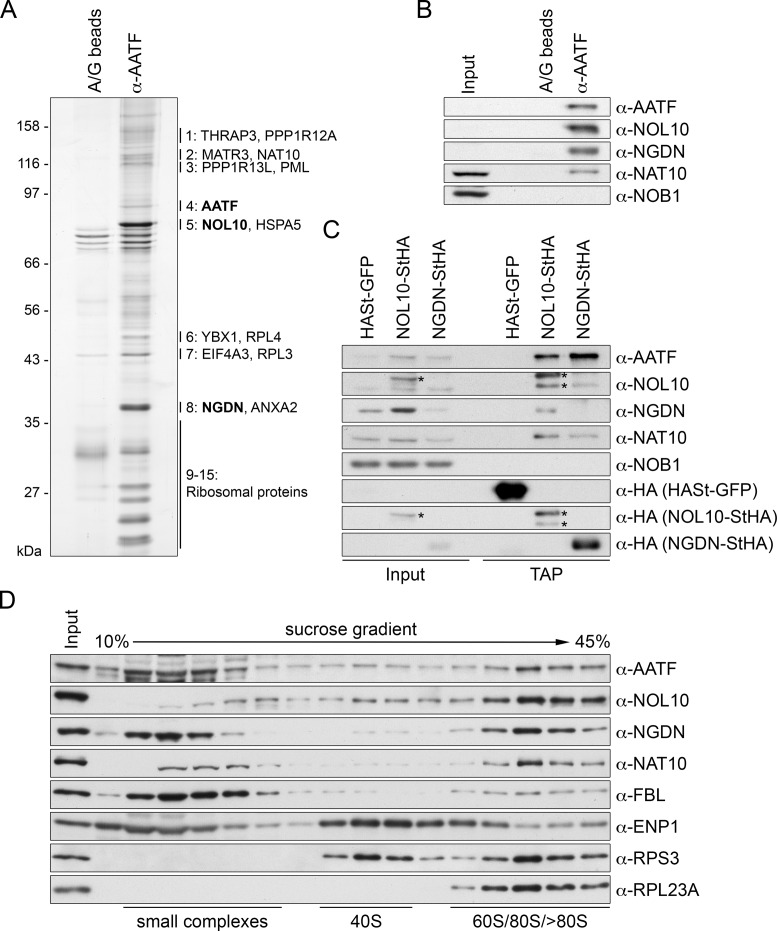
Figure 3.
Identification of NOL10 and NGDN in complex with AATF. (A) AATF was isolated by immunoprecipitation (IP) from HeLa cells using an affinity-purified antibody coupled to protein A/G beads. Beads without antibodies were used as negative control. Co-precipitated proteins were analyzed by SDS-PAGE followed by silver staining. Numbering next to the gel refers to MS analysis of excised bands from a Coomassie Blue-stained gel, which identified AATF, NOL10 and NGDN among the most abundant proteins at the AATF complex (Supplementary Table S1). For band 1 to 8, the two proteins with the highest peptide number detected are listed. Bands 9–15 contained predominantly ribosomal proteins. Note that AATF is inefficiently eluted from the antibody. (B) Western blot analysis of input (0.04%) and eluate (20%) samples from experiment in (A) confirms the strong enrichment of NOL10 and NGDN in the protein complex isolated by AATF IP. (C) Tandem affinity purification (TAP) of NOL10 and NGDN in complex with AATF. Strep-HA (StHA)-tagged NOL10, NGDN or HASt-tagged GFP were induced with tetracycline in HEK293 cell lines and cell extracts (input) subjected to TAP. Inputs (0.008%) and TAP eluates (20%) were analyzed by immunoblotting using the indicated antibodies. Asterisks indicate the StHA-tagged NOL10 bait protein and a degradation product recognized by the anti-NOL10 and anti-HA antibodies. (D) HeLa cell extract was separated by centrifugation on a linear 10–45% sucrose gradient. Proteins present in the input and gradient fractions were analyzed by immunoblotting.