Abstract
Background
Despite broad and intense conventional immunosuppression, long-term survival after lung transplantation lags behind that for other solid organ transplants, primarily because of allograft rejection. Therefore, new strategies to promote lung allograft acceptance are urgently needed. The purpose of the present study was to induce allograft tolerance with a protocol compatible with deceased donor organ utilization.
Methods
Using the MHC-mismatched mouse orthotopic lung transplant model, we investigated a conditioning regimen consisting of pretransplant T cell depletion, low dose total body irradiation and posttransplant (donor) bone marrow and splenocyte infusion followed by posttransplantation cyclophosphamide (PTTT-PTB/PTCy).
Results
Our results show that C57BL/6 recipients of BALB/c lung allografts undergoing this complete short-duration nonmyeloablative conditioning regimen had durable lung allograft acceptance. Mice that lacked 1 or more components of this regimen exhibited significant graft loss. Mechanistically, animals with lung allograft acceptance had established higher levels of donor chimerism, lymphocyte responses which were attenuated to donor antigens but maintained to third-party antigens, and clonal deletion of donor-reactive host Vβ T cells. Frequencies of Foxp3+ T regulatory cells were comparable in both surviving and rejected allografts implying that their perturbation was not a dominant cell-regulatory mechanism. Donor chimerism was indispensable for sustained tolerance, as evidenced by acute rejection of allografts in established chimeric recipients of PTTT-PTB/PTCy following a chimerism-ablating secondary recipient lymphocyte infusion.
Conclusion
Together, these data provide proof-of-concept for establishing lung allograft tolerance with tandem donor bone marrow transplantation (BMT) using a short-duration nonmyeloablative conditioning regimen and PTCy.
Introduction
Lung transplantation is the final therapeutic option for select patients with end-stage lung disease. Unfortunately, the longevity of transplanted lungs continues to be shorter than that of other solid organ allografts. Despite the use of broad immunosuppression therapies, episodes of acute and chronic cellular rejection are quite common1,2. Therefore, new strategies are needed to limit alloreactivity and promote lung allograft tolerance.
The establishment of stable multi-lineage donor chimerism has been shown to contribute to tolerance induction of transplanted solid organs3-5. The clinical translation of this strategy has been further stimulated by the growing success of nonmyeloablative regimens in patients with hematologic malignancies6. While early clinical attempts with nonmyeloablative allografting using HLA-mismatched donors were associated with a high risk of rejection, more recent studies suggest that modifications such as use of posttransplant cyclophosphamide (PTCy) can result in engraftment of HLA-mismatched, related BM (haploidentical) with low nonrelapse mortality and acceptable rates of acute and chronic GVHD7,8. Furthermore, using HLA-mismatched haploidentical donors after nonmyeloablative conditioning has shown that PTCy is an essential component of the strategy for the treatment of sickle cell disease and for successful combined BM and kidney transplantation9,10. While there is growing experience with combined BMT and solid organ transplantation, it is limited to either living related donors11 or haplo-identical12 donors. This is impractical for broad application in lung transplantation which mostly relies on the cadaveric donors4,12.
In this study, we used a mouse orthotopic left lung transplant model13,14 to test whether a conditioning regimen beginning 12 hours prior to lung transplantation would induce lung allograft tolerance. The nonmyeloablative conditioning strategy. consisting of pretransplantation total body irradiation and T cell depletion, simultaneous lung and bone marrow transplantation, and post transplantation cyclophosphamide administered 72 hours later (PTTT-PTB/PTCy), is based on our previously developed nonmyeloablative regimens15,16. We report that MHC-mismatched allograft recipients undergoing PTTT-PTB/PTCy showed significantly improved allograft acceptance. Our data suggests that a 12-hour, nonmyeloablative conditioning regimen prior to tandem MHC-disparate lung and bone marrow transplantation followed by PTCy has the potential for establishing functional lung acceptance.
Materials and Methods
Mice
C57BL/6 (C57BL/6, H-2b) and BALB/c (H-2d) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and housed under specific pathogen-free conditions before surgery, and open access conditions after surgery. The Johns Hopkins University Animal Care and Use Committee approved all animal care protocols.
Nonmyeloablative conditioning
Total body irradiation (TBI; 250 cGy, 137Cs irradiator, Gammacell 40; Atomic Energy of Canada) and T cell depletion with 2 mg of pan-T cell–depleting monoclonal antibody (anti-Thy1.2 mAb, intraperitoneal [i.p]., Bio X Cell; clone 30H12) were initiated 8-12 hours before lung implantation. HSCs comprised of murine bone marrow (BM; 2.5 × 107) cells supplemented with 3 × 107 unfractionated splenocytes were administered after orthotopic lung transplantation, unless otherwise indicated. BM cells were flushed from the hind-limb bones of BALB/c donors with RPMI 1640 containing 5% heat-inactivated fetal calf serum and 2mM EDTA. Spleens were mashed and passed through 70-μm cell strainers as a single-cell suspension. BM and spleen cells were combined and administered as a HSC infusion 2-6 h after completion of orthotopic lung transplantation. Cyclophosphamide (Baxter Healthcare, Deersfield, IL; 200 mg/kg) was administered i.p. 72h after completion of the lung transplantation15,16. Allogeneic lung transplantations were performed in the BALB/c to C57BL/6 strain combination.
Mouse orthotopic lung transplant and GVHD monitoring
Lungs were transplanted with a cuffed technique13,14,17. Donor mice were sedated with etomidate (1 mg, i.p.), intubated, and maintained on inhaled isoflurane until euthanized. Recipients were both initially sedated and maintained on inhaled isoflurane. Mice received subcutaneous buprenorphine (0.03-0.05 mg/kg) before extubation and every 6 h thereafter as needed. Unless otherwise specified, animals were sacrificed for analysis at 40-60 days posttransplant. GVHD monitoring were performed as previously described15,18.
Post-surgical care
Lung recipients were maintained in individual cages with food and water ad libitum. Cages were changed regularly, and animals were checked twice weekly. The vivarium allows open access and provides unfiltered air.
Results
Assessment of protocol conditioning components required for lung allograft acceptance
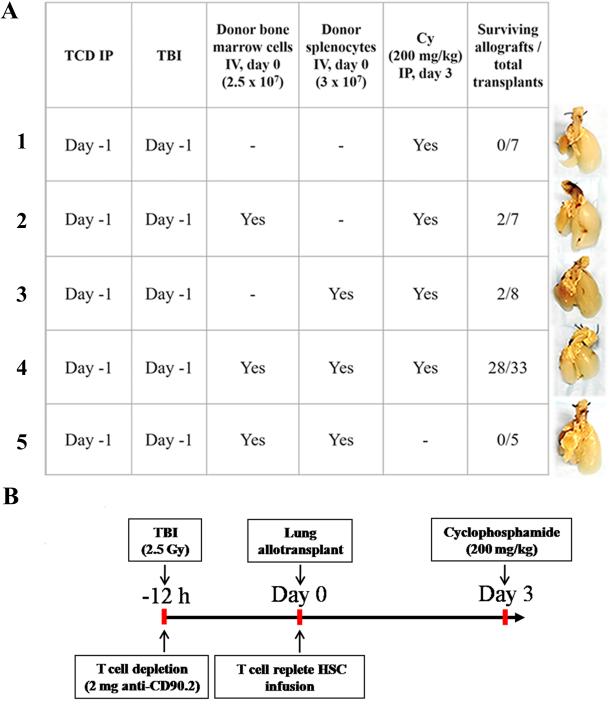
We hypothesized that successful induction of donor chimerism would promote acceptance of the lung allograft. We sought to establish lung allograft acceptance using a protocol where recipient conditioning was initiated 12 hours prior to lung implantation. Based on previous murine BMT studies15,19,20, we hypothesized that host preconditioning with T cell depletion in combination with low-dose TBI, administration of a clinically achievable dose of HSCs and PTCy would induce sufficient macrochimerism to promote orthotopic donor lung acceptance. Low dose TBI was administered to promote donor myeloid cell engraftment by creating space within the BM for donor cells to engraft15,16. Additionally, we depleted host T cells with anti-T cell antibody (anti-Thy 1.2 mAb) to reduce the total number of host-reactive T cells and protect against early rejection19,20. The donor HSC inoculum was comprised of BM and spleen cells as mouse BM, unlike human BM, is comparatively deficient in T cells. Figures 1A and S1 summarize the allograft survival results along with corresponding gross photographs of experiments where C57BL/6 mice treated with various conditioning regimens underwent donor BALB/c orthotopic lung transplantation. A regimen consisting of TBI conditioning, anti-Thy1.2, and PTCy without donor BALB/c HSC was insufficient to induce allograft survival (Figure 1A, Group 1). However, the addition of donor HSC inoculum to a TBI/anti-Thy1.2/PTCy regimen resulted in survival of 28/33 (85%) lung allografts (Figure 1A, Group 4). Interestingly, administration of either donor splenocytes alone or BM alone was insufficient to achieve allograft survival in more than 50% of mice (Figure 1A, Groups 2 and 3, respectively). This likely reflects the importance of cell dose and HSC inoculum composition for induction of tolerance with PTCy15,16,19,21. Consistent with previous work showing a critical role of PTCy, no allografts survived in mice that received TBI, anti-Thy1.2, and HSC without PTCy (Figure 1A, Group 5). We also explored whether post-surgical conditioning could induce stable allograft acceptance. Allografts survived in only 1 of 5 chimeras that received conditioning post-operatively (Figure S1), implying a critical requirement for preconditioning to be performed 8-12 hours prior to surgery in our model. Based on these results, Figure 1B schematically depicts the optimal regimen we used in all subsequent experiments.
Figure 1.
(A) Allograft survival requires T cell–replete hematopoietic stem cells (HSC) and posttransplant cyclophosphamide (PTCy). Representative gross pathology of left lung allograft corresponding to different experimental conditioning regimens with/without donor bone marrow, splenocytes and posttransplant cyclophosphamide. (B) Regimen for combined BM and orthotopic lung allograft transplantation (PTTT-PTB/PTCy). Recipient mice received 250 cGy total body irradiation (TBI) followed 15 minutes later by T cell depletion with anti-Thy 1.2 monoclonal antibody 8-12 h before lung transplantation. Two to 6 hours following lung implantation, they received 2.5 × 107 donor strain bone marrow cells and 3 × 107 spleen cells (donor T cell–replete hematopoietic stem cells [HSC]) by intravenously followed by intraperitoneal administration of cyclophosphamide (200 mg/kg) 72 h after completion of lung implantation
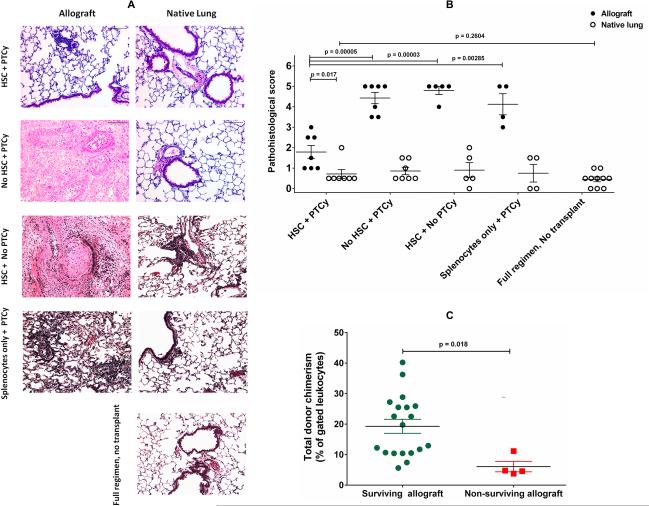
Lung allograft survival is associated with lack of histological signs of rejection but requires sustained donor chimerism
We next sought to determine: 1) the correlation between visual graft acceptance and histological findings; 2) the robustness of graft acceptance; and 3) the relationship between graft acceptance and donor chimerism. Histologic evaluation of lung allografts (BALB/c → C57BL/6) from mice conditioned with different experimental regimens are shown in Figure 2. On microscopic inspection, lung allografts from hosts conditioned with PTTT-PTB/PTCy showed only mild peribronchial, perivascular, and/or interstitial inflammation that was reflected in their pathohistologic scores being comparable to those of the native lungs (Figure 2A-B). It is important to note that while our results show a dramatic attenuation of allograft injury, we cannot exclude statistically insignificant differences between allograft and native lung. Allografts from mice that received TBI, T cell depletion, and PTCy, but no HSCs exhibited massive central hemorrhagic necrosis with some fibrosis, and a pathohistologic score significantly worse than that of allografts from mice receiving our complete regimen (Figure 2A-B). With either treatment, the native lung exhibited only mild peribronchial and/or perivascular inflammation (Figure 2A-B). Notably, all lung allografts harvested between 35 and 44 days after transplantation survived. This was also the case with allografts harvested 65-74 and 75-88 days after transplantation. When lung allografts did not survive, despite use of our full protocol, graft loss was most frequently observed at days 45-54 (71% survival), followed by days 55-64 (86% survival). Overall allograft survival was 28/33 (85%).To determine whether the level of donor chimerism was associated with lung allograft survival, we evaluated individual mice for the relationship between lung allograft survival/ nonsurvival state and the percentage of donor chimerism in peripheral blood at the time of lung harvest (Figure 2C). Compared with surviving lung allografts, nonsurviving lung allografts exhibited a significantly lower level of peripheral blood donor chimerism at the time of allograft harvest. To validate the causal relationship between donor chimerism and allograft survival, 1 group of PTTT-PTB/PTCy-treated mice with established chimerism 4 weeks following transplantation was given a chimerism-ablating secondary recipient lymphocyte infusion (RLI) comprised of host splenocytes. Two weeks post-RLI administration, the chimerism was ablated (data not shown) and the allografts were rejected (Figure S2 A-B). Cellular analyses revealed elevated levels of cytokine-producing host CD4+ T cells in the allograft (Figure S2 C-D). These results suggest there is a threshold level of donor chimerism that must be established and maintained to achieve durable lung allograft survival with our protocol.
Figure 2.
(A-B) Effect on day 60 histology of including T cell–replete hematopoietic stem cells (HSC) in conditioning protocol. (A) Representative lung histology with H&E-staining (20x) at day 60 and (B) comparison of mean pathohistologic scores (n = 4-10 per group) of lung allografts versus native lungs from BALB/c -> C57BL/6 transplants as well as preconditioned untransplanted mice. Mice received pretransplant irradiation, T cell depletion, and posttransplantation cyclophosphamide (PTCy) with or without donor T cell–replete donor hematopoietic stem cell (HSC) infusion. (C) Relationship between lung allograft acceptance and level of peripheral donor chimerism following PTTT-PTB/PTCy. Comparison of total donor chimerism as a percentage of gated leukocytes at time of harvest in surviving (n=19) and nonsurviving (n=4) lung allografts from recipients treated with PTTT-PTB/PTCy. Bars indicate mean values ± SEM.
Tolerant mice demonstrate donor-specific loss of alloreactivity and clonal deletion of reactive T cells
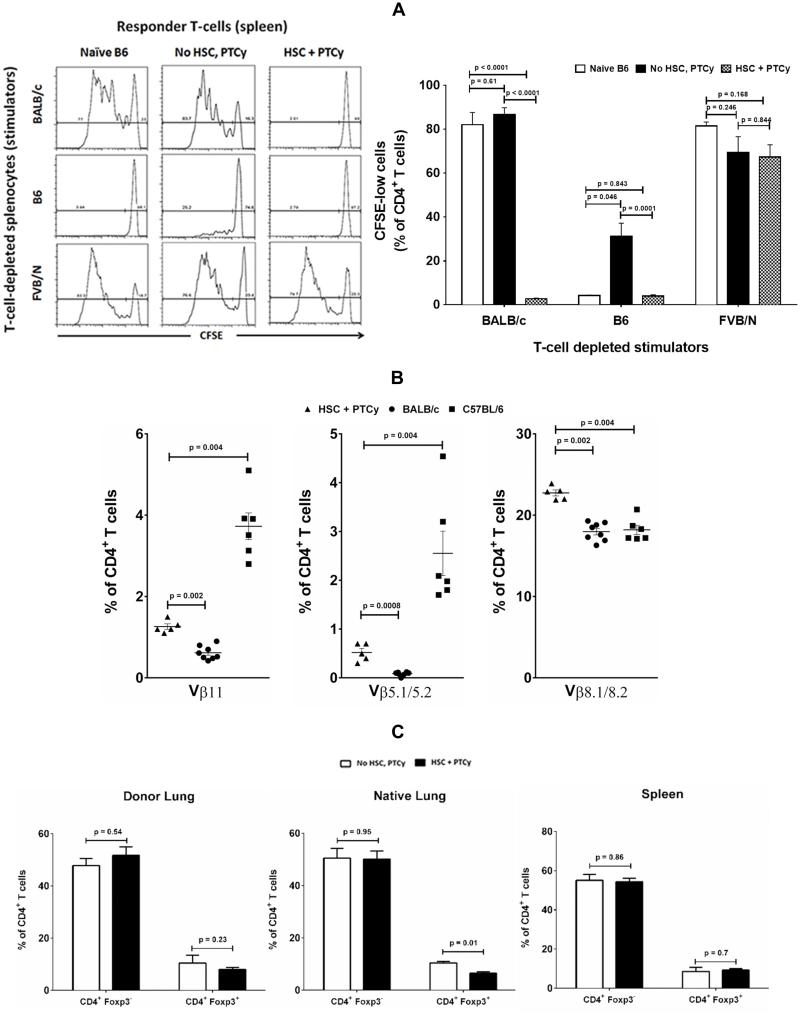
Next, mice with viable lung allografts were then evaluated for donor- and third party-alloreactivity in vitro (Figure 3A). Splenic CD4+ T cells from naïve C57BL/6 mice as well as HSC-excluded PTTT-PTB/PTCy-treated recipients of BALB/c lung allograft proliferated robustly when cultured with T cell–depleted splenocytes (stimulators) from donor (BALB/c) or third party (FVB/N) mice. By contrast, CD4+ T cells from PTTT-PTB/PTCy-treated C57BL/6 recipients with surviving BALB/c lung allografts proliferated poorly only with donor alloantigens but with T cell–depleted splenocytes from donor (BALB/c) mice but did retain their proliferative response to stimulators from third-party (FVB/N) mice. These findings suggest that PTTT-PTB/PTCy tolerizes recipient mice by preferentially targeting donor-antigen-specific T cells without otherwise compromising immune competence.
Figure 3.
(A) Donor-specific loss of alloreactivity in lung allograft-accepting mixed chimeras. Lung allograft-tolerant mixed chimeras were evaluated for donor specificity of their tolerance in an in vitro mixed lymphocyte reaction. Splenic CD4+ T cells were isolated from hosts that received either no prior treatment (C57BL/6 untransplanted) or prior lung transplantation (BALBc->C57BL/6) and PTTT-PTB/PTCy with or without T cell–replete donor hematopoietic stem cells (HSC). The splenic CD4+ T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and co-cultured with T cell–depleted splenocytes from naïve C57BL/6 mice, naïve BALB/c mice, or naive FVB/N mice. Proliferation patterns, indicated by progressive dilution of CFSE fluorescence intensity with every cell division, were evaluated. Data are representative of 3 independent experiments. Proliferation was quantified in terms of CFSE-low (divided) cell frequency within the gated CD4+ subset and expressed as mean ± SEM.
(B) Deletion of donor-reactive Vβ11 and Vβ5.1/5.2 CD4+ T cells with preservation of Vβ8.1/8.2 CD4+ T cells in peripheral blood at harvest in lung allograft-tolerant C57BL/6 mice that underwent PTTT-PTB/PTCy. Expression of Vβ11, Vβ5.1/5.2, and Vβ8.1/8.2 in peripheral CD4+ T cells of naïve donor (BALB/c), naïve recipient (C57BL/6), and the H-2Kb+ (host) subset of BALB/c->C57BL/6 lung allograft–accepting mice that received PTTT-PTB/PTCy. Vβ staining was analyzed by flow cytometry. Data are means ± SEM of 5-8 separate experiments in each group.
(C) Comparable H-2Kb+ CD4+ effector (Foxp3−) and T regulatory (Foxp3+) frequencies in mice with surviving and rejected allografts. Lung allograft, native lung and spleen from PTTT-PTB/PTCy or PTTT-PTB/PTCy minus HSC groups were dissociated into single cell suspensions. Cells were surface-stained for H-2Kb, CD3 and CD4, fixed and permeabilized before staining for intranuclear Foxp3. Data are means ± SEM of 5-6 separate experiments in each group.
We 15 and others 22,23 have shown that clonal deletion contributes to the tolerance induced by other cyclophosphamide-based nonmyeloablative regimens. Vβ11+ and Vβ5.1/5.2+ T cells are known to be clonally deleted in BALB/c mice, but not in C57BL/6 mice because of the presence of an endogenous, retrovirally encoded superantigen 22,24, whereas Vβ8.1+ T cells are maintained in both of these strains. Therefore, we evaluated whether clonal deletion was associated with PTTT-PTB/PTCy-induced tolerance. As shown in Figure 3B, we confirmed the clonal deletion of Vβ11+ and Vβ5.1/5.2+ families in CD4+ T cells of control donor BALB/c mice and the retention of these clones in T cells of naïve control C57BL/6 mice while both strains expressed the Vβ8.1+ clone. In contrast, both Vβ11+ and Vβ5.1/5.2+ H-2Kb+ (host) CD4+ T cells were significantly reduced in the peripheral blood of chimeric C57BL/6 mice that received tandem BMT and lung allografts from BALB/c mice. We also examined the role of CD4+ Foxp3+ T regulatory cells (Tregs) in tolerance-induction by PTTT-PTB/PTCy by examining their composition in chimeras on day 60 posttransplant. Interestingly, both surviving allografts from PTTT-PTB/PTCy treated mice and rejected allografts from PTTT-PTB/PTCy minus HSC treated mice, the frequencies of Tregs in host CD4+ T cells from allograft, native lungs and spleen were comparable (Figure 3C). This suggests that major Treg-number perturbation is not a dominant mechanism of organ acceptance in this model. Additional studies are required to fully dissect the importance of both clonal deletion of donor-reactive host T cells and early expansion of Foxp3+ Tregs seen in alloBMT models (39) in tolerance induction with PTTT-PTB/PTCy regimen.
Discussion
Here, we show for the first time the establishment of long-term lung allograft acceptance across major MHC barriers, using a protocol compatible with deceased donor organ use. Our studies suggest that all components (low-dose TBI, T cell depletion, donor HSCs plus splenocytes, and PTCy) of our protocol (PTTT-PTB/PTCy) are required to establish allograft tolerance, as experiments lacking any of these components resulted in lung rejection. Stable chimerism appears to be essential for long-term lung acceptance, suggesting that transient engraftment is not sufficient in this model. Our results suggest that the capacity of PTCy to modulate alloreactivity and promote bone marrow tolerance as part of a nonmyeloablative conditioning regimen can be used as a tool to achieve acceptance of transplanted solid organs through the establishment of chimerism. Additionally, our experimental results indicate that this protocol can be implemented within the short timeframe of lung transplant and achieve durable acceptance out to 3 months of follow-up.
Our results support the concept that cytotoxic destruction of alloreactive T-effectors by the addition of PTCy25, plays an important role in establishing hematopoietic chimerism and tolerance to the lung allograft. Cyclophosphamide is a pro-drug26 whose cytotoxicity is dependent on conversion to aldophosphamide. Aldophosphamide is, in turn, inactivated by aldehyde dehydrogenase (ALDH)27. Because ALDH is absent in activated, replicating effector T cells28-30, alloreactive lymphocytes are highly susceptible to deletion8,31. In contrast, stem cells and regulatory T cells with higher ALDH levels are resistant30,32. Finally, PTCy allows the preservation of the nonalloantigen responsive and nonproliferative, pathogen-specific memory T cells which are important for host defense33.
A key aspect of functional lung allograft acceptance that has emerged from our model is that the maintenance of donor chimerism represents a potential biomarker of a functional allograft. Our data also suggest that a potential threshold level of donor hematopoietic chimerism may be important, as lung allografts were rejected both in mice that had lower levels of donor chimerism as in mice whose established chimerism was ablated with RLI. These findings are consistent with previous studies that have shown that a substantial level of donor hematopoietic chimerism is required for functional allograft acceptance34,35. In our model, stable donor chimerism appears to be essential for maintaining functional lung acceptance. This is in contrast with kidney allografts, where loss of donor chimerism after combined allografting does not lead to compromise in allograft function36,37 .
Our study demonstrates that lung allograft acceptance is also associated with unresponsiveness to donor allo-antigens. Encouragingly, transplanted animals maintained functional responsiveness to third party allo-antigens, implying that broad immunologic competence was not affected. Further analysis revealed a reduction of specific Vβ populations in C57BL/6 recipients of BALB/c allografts, suggesting that clonal deletion is a major mechanism of tolerance to donor antigens. Taken together, these findings suggest that the establishment of donor chimerism, along with clonal deletion leading to reduced host alloreactivity, is critical for long-term lung allograft viability. However, further studies are needed to fully comprehend the role of Foxp3+ Tregs especially in early phases of tolerance induction using this regimen.
There are several caveats to our studies. First, because we used nonspecific T cell depletion, the extent to which host and donor T cell depletion with anti-Thy mAb plays a role is unclear. The addition of ATG in the clinic was essential for engraftment in sickle cell patients10, thus some level of pan-T cell depletion may be essential. The use of T cell depletion could potentially increase the risk of viral infection. One therefore needs to balance the potential benefit of pan-T cell depletion on engraftment with the increased risk of infection when translating these therapeutic concepts to humans. Second, PTCy may not be sufficient as a single agent to prevent allograft rejection in the clinic after HLA-mismatched allografting. We have previously shown, in a murine BMT model, that sirolimus may be synergistic with PTCy in promoting donor chimerism38. This synergy was not seen when sirolimus was replaced with cyclosporine. Finally, we acknowledge that other mechanisms may be important for a PTCy-based regimen to establish tolerance. For example, T-regulatory cells appear to be resilient and contribute to tolerance via expression of high protective levels of ALDH18,32. Although the role of Tregs in this model appears to be limited, they may very well contribute synergistically to the induction of antigen-specific tolerance early after allografting. Third, although chimerism was low in our model, various modifications, such as adding pretransplant fludarabine or achieving more myeloablation through addition of busulfan, can be applied to increase donor hematopoietic chimerism8,15,35.
In summary, we have developed a 12-hour protocol that induces lung allograft tolerance across stringent MHC barriers. However, significant modifications to this approach are required prior to its clinical translation specifically in relation to the timing of conditioning administration, deceased donor lung procurement and allografting. Furthermore, the potential toxicity of conditioning on patients with end stage lung-disease would need to be strongly considered. Encouragingly, these same regimens are now routinely used in patients with hematological malignancies up to the 7th decade39. Additionally, new strategies for allograft preservation in a transportable, warm-perfused, ventilated system that extend the time between lung harvest and implantation up to 12 hours without deleterious sequelae are being developed40. Although we have demonstrated efficacy of PTTT-PTB/PTCy in experimental orthotopic lung transplantation, additional studies, preferentially in large animal models, are needed to further define the optimal timing of pretransplant T cell depletion and total body irradiation and ultimately human translation.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Clare Levine, MS, ELS, for her superb editing of this manuscript.
Grant support: Supported by: R01CA122779 (LL)
Abbreviations
- BM
bone marrow
- BMT
bone marrow transplantation
- CFSE
carboxyfluorescein succinimidyl ester
- GVHD
graft-versus-host disease
- HSC
hematopoietic stem cell
- PTCy
posttransplant cyclophosphamide
- TBI
total body irradiation
Footnotes
Additional methods are described in supplemental information.
References
- 1.Martinu T, Howell DN, Palmer SM. Acute cellular rejection and humoral sensitization in lung transplant recipients. Semin Respir Crit Care Med. 2010;31(2):179–188. doi: 10.1055/s-0030-1249113. [DOI] [PubMed] [Google Scholar]
- 2.Weigt SS, Wallace WD, Derhovanessian A, et al. Chronic allograft rejection: epidemiology, diagnosis, pathogenesis, and treatment. Semin Respir Crit Care Med. 2010;31(2):189–207. doi: 10.1055/s-0030-1249116. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Leventhal J, Madsen JC, Strober S, Turka LA, Wood KJ. Tolerance: one transplant for life. Transplantation. 2014;98(2):117–121. doi: 10.1097/TP.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald-Hyman C, Turka LA, Blazar BR. Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation. Sci Transl Med. 2015;7(280):280rv282. doi: 10.1126/scitranslmed.aaa6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sachs DH, Sykes M, Kawai T, Cosimi AB. Immuno-intervention for the induction of transplantation tolerance through mixed chimerism. Semin Immunol. 2011;23(3):165–173. doi: 10.1016/j.smim.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101(4):1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 7.Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res. 2010;47(1-3):65–77. doi: 10.1007/s12026-009-8139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luznik L, O'Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Seminars in oncology. 2012;39(6):683–693. doi: 10.1053/j.seminoncol.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012:4. doi: 10.1126/scitranslmed.3003509. 56836dd8-7297-51aa-c941-a6c6ad96ddec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolanos-Meade J, Fuchs EJ, Luznik L, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120(22):4285–4291. doi: 10.1182/blood-2012-07-438408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358(4):362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 12.Szabolcs P, Buckley RH, Davis RD, et al. Tolerance and immunity after sequential lung and bone marrow transplantation from an unrelated cadaveric donor. J Allergy Clin Immunol Pract. 2015;135(2):567–570. doi: 10.1016/j.jaci.2014.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodd-o JM, Lendermon EA, Miller HL, et al. CD154 blockade abrogates allospecific responses and enhances CD4(+) regulatory T-cells in mouse orthotopic lung transplant. Am J Transplant. 2011;11(9):1815–1824. doi: 10.1111/j.1600-6143.2011.03623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lendermon EA, Dodd-o J, Coon TA, et al. CD8IL-17 T Cells Mediate Neutrophilic Airway Obliteration in T-bet Deficient Mouse Lung Allograft Recipients. Am J Respir Cell Mol Biol. 2015;52(5):622–33. doi: 10.1165/rcmb.2014-0059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luznik L. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98(12):3456–3464. doi: 10.1182/blood.v98.12.3456. [DOI] [PubMed] [Google Scholar]
- 16.Luznik L, Engstrom LW, Iannone R, Fuchs EJ. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complex-identical allogeneic marrow in mice conditioned with low-dose total body irradiation. Biol Blood Marrow Transplant. 2002;8(3):131–138. doi: 10.1053/bbmt.2002.v8.pm11939602. [DOI] [PubMed] [Google Scholar]
- 17.Okazaki M, Krupnick AS, Kornfeld CG, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007;7(6):1672–1679. doi: 10.1111/j.1600-6143.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 18.Ganguly S, Ross DB, Panoskaltsis-Mortari A, et al. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood. 2014;124(13):2131–2141. doi: 10.1182/blood-2013-10-525873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colson YL, Li H, Boggs SS, Patrene KD, Johnson PC, Ildstad ST. Durable mixed allogeneic chimerism and tolerance by a nonlethal radiation-based cytoreductive approach. J Immunol. 1996;157(7):2820–2829. [PubMed] [Google Scholar]
- 20.Mayumi H, Good RA. Induction of tolerance across major barriers using a two-step method with genetic analysis of tolerance induction. Immunobiology. 1989;179(1):86–108. doi: 10.1016/S0171-2985(89)80009-9. [DOI] [PubMed] [Google Scholar]
- 21.Durakovic N, Radojcic V, Skarica M, et al. Factors governing the activation of adoptively transferred donor T cells infused after allogeneic bone marrow transplantation in the mouse. Blood. 2007;109(10):4564–4574. doi: 10.1182/blood-2006-09-048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eto M, Mayumi H, Tomita Y, Yoshikai Y, Nomoto K. Intrathymic clonal deletion of V beta 6+ T cells in cyclophosphamide-induced tolerance to H-2-compatible, Mls-disparate antigens. J Exp Med. 1990;171(1):97–113. doi: 10.1084/jem.171.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomita Y, Ayukawa K, Yoshikai Y, Nomoto K. Mechanisms of cyclophosphamide-induced tolerance to IE-encoded alloantigens--evidence of clonal deletion in MHC antigen-reactive cells for skin allograft rejection. Transplantation. 1992;53(3):602–612. doi: 10.1097/00007890-199203000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Kappler JW, Staerz U, White J, Marrack PC. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- 25.Mayumi H, Himeno K, Tokuda N, Nomoto K. Drug-induced tolerance to allografts in mice. VIII. Effects of thymectomy and/or splenectomy on tolerance induction in an H-2-haplotype-identical strain combination. Transplantation. 1985;40(4):438–441. [PubMed] [Google Scholar]
- 26.Cohen JL, Jao JY. Enzymatic basis of cyclophosphamide activation by hepatic microsomes of the rat. J Pharmacol Exp Ther. 1970;174(2):206–210. [PubMed] [Google Scholar]
- 27.Sladek NE, Kollander R, Sreerama L, Kiang DT. Cellular levels of aldehyde dehydrogenases (ALDH1A1 and ALDH3A1) as predictors of therapeutic responses to cyclophosphamide-based chemotherapy of breast cancer: a retrospective study. Rational individualization of oxazaphosphorine-based cancer chemotherapeutic regimens. Cancer Chemother Pharmacol. 2002;49(4):309–321. doi: 10.1007/s00280-001-0412-4. [DOI] [PubMed] [Google Scholar]
- 28.Hilton J. Role of aldehyde dehydrogenase in cyclophosphamide-resistant L1210 leukemia. Cancer Res. 1984;44(11):5156–5160. [PubMed] [Google Scholar]
- 29.Jones RJ, Barber JP, Vala MS, et al. Assessment of aldehyde dehydrogenase in viable cells. Blood. 1995;85(10):2742–2746. [PubMed] [Google Scholar]
- 30.Kastan MB, Schlaffer E, Russo JE, Colvin OM, Civin CI, Hilton J. Direct demonstration of elevated aldehyde dehydrogenase in human hematopoietic progenitor cells. Blood. 1990;75(10):1947–1950. [PubMed] [Google Scholar]
- 31.Aisenberg AC, Wilkes B. Immunological tolerance induced by cyclophosphamide assayed by plaque spleen cell method. Nature. 1967;213(5075):498–499. doi: 10.1038/213498a0. [DOI] [PubMed] [Google Scholar]
- 32.Kanakry CG, Ganguly S, Zahurak M, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5(211):211ra157. doi: 10.1126/scitranslmed.3006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross D, Jones M, Komanduri K, Levy RB. Antigen and lymphopenia-driven donor T cells are differentially diminished by post-transplantation administration of cyclophosphamide after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19(10):1430–1438. doi: 10.1016/j.bbmt.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo Z, Wu T, Sozen H, et al. A substantial level of donor hematopoietic chimerism is required to protect donor-specific islet grafts in diabetic NOD mice. Transplantation. 2003;75(7):909–915. doi: 10.1097/01.TP.0000057832.92231.F5. [DOI] [PubMed] [Google Scholar]
- 35.Leong LY, Qin S, Cobbold SP, Waldmann H. Classical transplantation tolerance in the adult: the interaction between myeloablation and immunosuppression. Euro J Immunol. 1992;22(11):2825–2830. doi: 10.1002/eji.1830221111. [DOI] [PubMed] [Google Scholar]
- 36.Graves SS, Mathes DW, Georges GE, et al. Long-term tolerance to kidney allografts after induced rejection of donor hematopoietic chimerism in a preclinical canine model. Transplantation. 2012;94(6):562–568. doi: 10.1097/TP.0b013e3182646bf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawai T, Sachs DH, Sykes M, Cosimi AB. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2013;368(19):1850–1852. doi: 10.1056/NEJMc1213779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzhugh CD, Weitzel RP, Hsieh MM, et al. Sirolimus and post transplant Cy synergistically maintain mixed chimerism in a mismatched murine model. Biol Blood Marrow Transplant. 2013;48(10):1335–1341. doi: 10.1038/bmt.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kasamon YL, Bolanos-Meade J, Prince GT, et al. Outcomes of Nonmyeloablative HLA-Haploidentical Blood or Marrow Transplantation With High-Dose Post-Transplantation Cyclophosphamide in Older Adults. J Clin Oncol. 2015;33(28):3152–3161. doi: 10.1200/JCO.2014.60.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warnecke G, Van Raemdonck D, Smith M, Kukreja J, Loor G, Rea F. The Organ Care System (OCS™) Lung INSPIRE International Trial Results. J Heart Transplantation. 2015;34(4S):S96. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.