Figure 2. Disruption of the Binding of Talin to β1 Integrin, but Not to β3 Integrin, by B7-1.
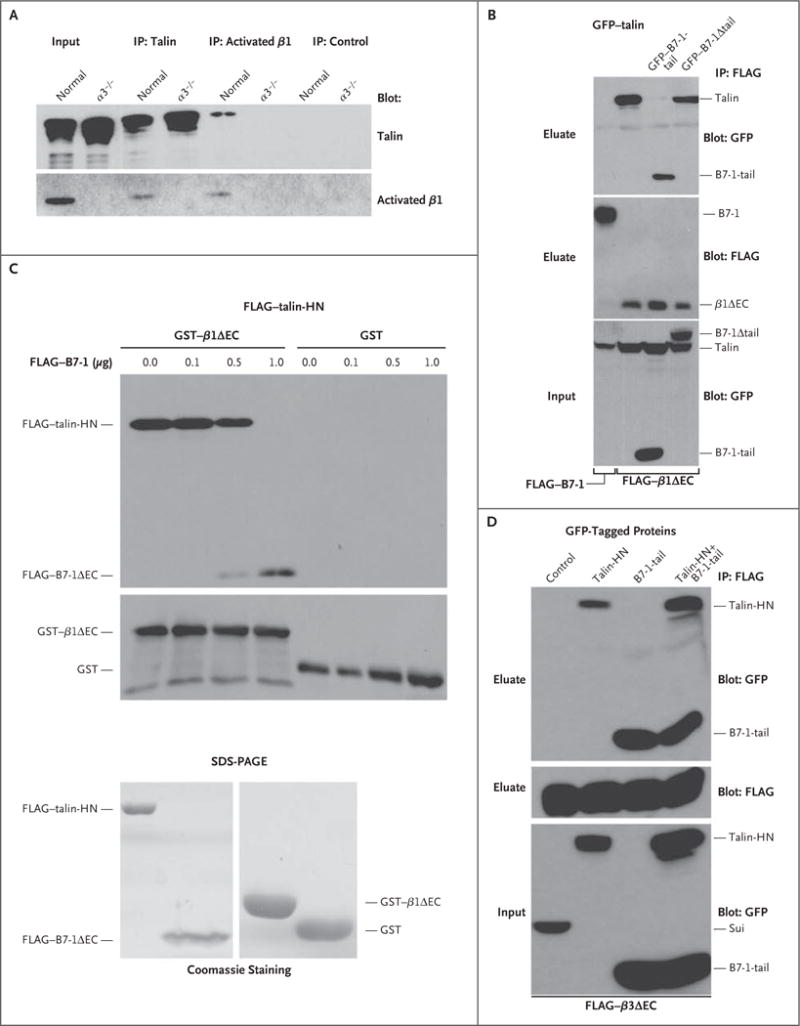
As shown in Panel A, endogenous talin coprecipitated with activated β1 integrin in normal podocytes but not in α3−/− podocytes. Immunoprecipitation (IP) with anti–green fluorescent protein (GFP) antibody served as a negative control. Input refers to protein extracts that served as starting material from which endogenous proteins were immunoprecipitated. As shown in Panel B, FLAG–B7-1 did not bind to talin (GFP–talin-HN, left lane); HN denotes head N-terminal domain. GFP–B7-1-tail but not GFP–B7-1Δ tail blocked the interaction of talin (GFP–talin-HN) with β1 integrin (FLAG–β1ΔEC) in cotransfected HEK293 cells. Instead, B7-1-tail coprecipitated with β1 integrin. As shown at the top of Panel C, immobilized β1 integrin (GST–β1ΔEC) but not the GST control bound directly to purified talin (FLAG–talin-HN). In the presence of increasing amounts of FLAG–B7-1ΔEC, the binding of talin-HN to GST–β1ΔEC was gradually lost, whereas the binding of B7-1 to β1 integrin could be detected. As shown at the bottom of Panel C, Coomassie-stained sodium dodecyl sulfate–polyacrylamide-gel electrophoresis (SDS-PAGE) analysis showed the purity of recombinant proteins. As shown in Panel D, co-expression of GFP–B7-1-tail did not block the interaction of talin (GFP–talin-HN) with β3 integrin (FLAG–β3ΔEC) in triple-transfected HEK293 cells. Instead, both GFP–B7-1-tail and GFP–talin-HN coprecipitated with FLAG–β3ΔEC. No binding was found with the fusion protein GFP–sui (negative control).
