Abstract
Escherichia coli biotype O104:H4 recently caused the deadliest E. coli outbreak ever reported. Based on prior results, it was hypothesized that compounds inhibiting biofilm formation by O104:H4 would reduce its pathogenesis. The nonionic surfactants polysorbate 80 (PS80) and polysorbate 20 (PS20) were found to reduce biofilms by ≥ 90% at submicromolar concentrations and elicited nearly complete dispersal of preformed biofilms. PS80 did not significantly impact in vivo colonization in a mouse infection model; however, mice treated with PS80 exhibited almost no intestinal inflammation or tissue damage while untreated mice exhibited robust pathology. As PS20 and PS80 are classified as “Generally Recognized as Safe” (GRAS) compounds by the FDA, these compounds have clinical potential to treat future O104:H4 outbreaks.
Keywords: Escherichia coli, biofilm, polysorbate, O104:H4
Introduction
Escherichia coli O104:H4 (hereafter referred to as O104:H4) is a newly evolved pathogenic strain of E. coli responsible for a massive 2011 European outbreak (Mellmann et al. 2011). Genome sequencing of O104:H4 revealed that this strain evolved from an enteroaggregative E. coli (EAEC) that acquired an E. coli O157:H7 Stx2 phage (Mellmann et al. 2011). The 2011 outbreak was the most severe E. coli outbreak ever recorded, resulting in nearly 4,000 infections leading to 54 deaths. Over 22% of patients exhibited hemolytic uremic syndrome (HUS) suggesting that this E. coli isolate persists and expresses high levels of Stx toxin during infection that can enter the bloodstream and damage the kidneys (Beutin and Martin 2012, Bielaszewska et al. 2011, Frank et al. 2011). Since O104:H4 is resistant to many clinical antibiotics and antibiotics have been implicated with increased disease severity in Stx-producing E. coli infections (Bielaszewska et al. 2012), treating O104:H4 infections is difficult, necessitating the need for novel intervention strategies (Rahal et al. 2015).
O104:H4 harbors a number of virulence factors including but not limited to the pAA plasmid encoding the aggregative adherence fimbriae (AAF), two distinct operons encoding long polar fimbriae (lpf), and a Stx2-producing Shiga toxin lamboid phage (Mellmann et al. 2011, Ross et al. 2015). It is hypothesized that the high colonization ability of the EAEC parent strain combined with toxin production by the Stx2-phage accounts for the high numbers of infections and HUS rate (Steiner 2014). However, the specific O104:H4 virulence factors responsible for these disease outcomes requires further investigation.
Mouse infection models have implicated the siderophore aerobactin (Torres et al. 2012), lpf (Ross et al. 2015), biofilm formation (Al Safadi et al. 2012), and Stx2 production (Zangari et al. 2013) as important for disease. Moreover, naturally evolved O104:H4 strains lacking the pAA plasmid were isolated that correlated with less severe disease symptoms (Zhang et al. 2013). The importance of the pAA plasmid in O104:H4 colonization was recently questioned as it had no significant impact in the colonization or disease symptoms in an infant rabbit model, but this model does not exhibit HUS (Munera et al. 2014, Ross et al. 2015). These same studies determined that Stx2 is critical for disease and autotransporters and Lpf drive colonization.
Biofilm formation, defined as a multi-cellular community of microorganisms encased in an extracellular matrix, of O104:H4 has been implicated as an important driver of severe sequela. The pAA plasmid and the AAF were critical for in vitro biofilm formation and promoted increased adherence to cultured epithelial cells (Boisen et al. 2014). One outcome of this adherence was increased transit of the Stx2 toxin across the epithelial cell barrier (Boisen et al. 2014). Deletion of lfp1 fimbrial loci severely inhibited in vitro biofilm formation and adherence to both polarized and non-polarized epithelial cells and this mutation had an impact on in vivo colonization (Ross et al. 2015). In addition, infection of germ-free mice revealed the characteristic EAEC “stacked brick” morphology of O104:H4 in close proximity to the intestinal epithelium (Al Safadi et al. 2012), which was also observed in other studies (Munera et al. 2014, Torres et al. 2012, Zangari et al. 2013). This same study described a correlation between in vivo toxin expression and the induction of biofilm genes, leading to the hypothesis that in vivo biofilm formation of O104:H4 promotes toxin expression for unknown reasons (Al Safadi et al. 2012).
Since biofilms are implicated in the pathogenesis of O104:H4, the present study sought to identify new compounds that inhibit biofilm formation by O104:H4. It was shown that the common food additives polysorbate 80 (PS80) and polysorbate 20 (PS20) inhibit and disperse O104:H4 biofilms in vitro at sub-micromolar concentrations without negatively impacting growth. Although PS80 had no significant impact on O104:H4 colonization levels in a mouse infection model, this compound completely abolished intestinal inflammation and tissue damage compared with the untreated control. This research suggests that inhibiting O104:H4 biofilm formation is an attractive strategy to reduce the severity of these infections and identifies polysorbates as a potential treatment for future O104:H4 outbreaks.
Materials and methods
Bacterial strains and culture conditions
E. coli O104:H4 strain TW16133 (Al Safadi et al. 2012) was used in all in vitro experiments. O104:H4 was grown in Luria-Bertani (LB) medium (Accumedia, Lansing, MI) incubated at 35°C shaking at 220 RPM. For in vivo mouse studies the bioluminescent O104:H4 strain RJC001 was used (Torres et al. 2012).
In vitro biofilm assays
Biofilms were measured by staining with 0.41% crystal violet (CV) solubilized in 12% ethanol in 96-well microtiter plates. O104:H4 was inoculated 1 to 500 into LB medium from turbid overnight cultures and 160 μl of this suspension were placed into wells of clear 96 well polystyrene CellStar microtiter plates (Greiner Bio-One, Monroe, NC). Cultures were grown at 35°C with rotation for 8 h. The plate was washed with 200 μl of phosphate buffered saline (PBS). Subsequently 200 μl of 95% ethanol were added for 10 min at room temperature to fix the cells. The ethanol was tapped out of the 96 well plate and 200 μl of CV solution were added and allowed to incubate at room temperature for 2 min. The CV solution was tapped out of the 96 well plate and then the plate was washed with tap water 3 times to remove CV not bound in biofilms. The water was removed by tapping and 200 μl of ethanol were added to each well to elute the CV from the biofilms. The eluted CV was then measured on a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA) at 595 nm. When necessary, 1:10 dilutions of the CV solution were analyzed to prevent absorbance saturation. Bacterial dispersion from biofilms was measured in the same way as in vitro biofilm assays by CV staining with replicated microtiter plates. One plate was fixed with ethanol at 5 h while polysorbates were added to the other plate at 5 h then allowed to incubate for 3 furthe hours before biofilms were measured.
Bacterial attachment assay
Turbid overnight cultures of O104:H4 were diluted 1 to 62.5 into fresh medium with 0.01% polysorbates in treated samples. 100 μl of this solution were added to each well of a 96 well microtiter plate. Bacteria were allowed to attach duringincubation for 1 h at 35°C with shaking at 100 rpm in a Syrotory Shaker Model G76 (New Brunswick Scientific, Edison, NJ). Wells were washed 2 times with 150 μl of PBS to remove planktonic cells that were unattached and stained with 150 μl of SYTO 9 (Invitrogen, Waltham, MA) diluted 1:1000 in PBS for 15 min. Wells were washed again with 150 μl of PBS then fixed with 150 μl of 3.7% formaldehyde. Cells were enumerated by counting three fields of view in six wells per sample on a Nikon Eclipse TS100 equipped with X-Cite series 120 Q illuminator (Exfo) inverted epi florescent microscope.
In vivo bacterial infections in mice
Eight to ten week old female CD-1 (ICR) mice were purchased from Charles River Laboratories (Willimington, MA). Animals were housed in a specific pathogen-free barrier under Biosafety Level 2 conditions. 48 h before the infection, mice were treated with streptomycin (5 g l−1 in their drinking water supplemented with 6.7% fructose) to reduce the normal flora (Torres et al. 2012). The level of water consumption by mice was similar across all cages evaluated. Food was restricted for 12 h prior to infection and cimetidine was also administered at a concentration of 50 mg Kg−1 of body weight 2 h prior to infection to reduce the acidity of the stomach. All animal studies were performed in accordance with the Animal Care and Use Committee’s guidelines at UTMB as recommended by the National Institute of Health (NIH).
Bacterial infection and treatment
For the bioluminescence experiments, animals were inoculated with a suspension of bioluminescent O104:H4 RJC001 as previously described (Torres et al. 2012). The strain RJC001 is resistant to 100 μg ml−1 of streptomycin and 50 μg/ml of kanamycin. A total of 12 mice were infected with the strain RJC001 at a bacterial suspension of 1× 108 CFU resuspended in 400 μl of PBS via oral gavage as previously described (Torres et al. 2012). The infected mice were divided in two groups of six mice each.
The group used to evaluate the effect of PS80 (Sigma Aldrich, St Louis, MO) on O104:H4 infection was treated by diluting 0.01% of PS80 in their drinking water throughout the experiment. The remaining group of six mice was untreated and used to monitor the regular course of infection by O104:H4 strain RJC001. There was no difference in the volume of water consumed by the treated and untreated groups.
Bioluminescent quantification of infection
For in vivo imaging, mice were anesthetized with 2–3% isoflurane in an oxygen-filled induction chamber. Once anesthetized, the mice were transferred to the in-chamber anesthesia delivery system where they were imaged. Bioluminescent images were acquired by using an IVIS Spectrum (Caliper Corp., Alameda, CA). A bioluminescent signal is represented in the images with a pseudo-color scale ranging from red (most intense) to violet (least intense) indicating the intensity of the signal. Signal intensities were obtained from regions of interest (ROIs), which are user-specified areas in an optical image. ROI of same size were drawn at the mouse abdominal cavity, and the same ROI was used for each time point evaluated to be able to establish a comparison between groups and different days. Signal from ROIs were expressed as photon flux (photons s−1 cm−2 steradian), where steradian (sr) refers to the photons emitted from a unit solid angle of a sphere. Any value outside the scale ranging from 1.1×108 to 8×105 (photons s−1 cm−2 steradian) was not used, since they fell outside the limit of detection (Torres et al. Popov 2012). All mice were monitored daily up to 6 days post-infection.
In order to correlate the bioluminescence readouts with bacterial counts, the number of bacteria was monitored in fecal pellets at 1, 2, 4 and 6 days post-infection. Feces were resuspended in PBS by vortexing, and the bacteria were plated for enumeration. For quantification of bacteria in tissues, sections of the cecum were collected at 6 days post-infection in 15 ml tubes containing PBS and homogenized using the Covidien Precision Disposable Tissue Grinder Systems. The resuspended feces and tissue homogenates were then serially diluted and plated on MacConkey agar containing streptomycin (100 μg ml−1) and kanamycin (50 μg ml−1). After overnight incubation at 37 °C, colonies were counted and expressed as either CFU g−1 of feces or CFU organ−1.
Histopathology
Sections of mouse terminal small intestine (ileum), and cecum were excised at 6 days post-infection and washed with PBS. The sections were fixed in buffered 10% formalin, paraffin-embedded, sectioned into 5 μm slices and then stained with hematoxylin and eosin at the Histopathology Core at UTMB. The tissues were examined and scored by a pathologist that was completely blinded to any details of the study. Tissues were scored according to degrees of severity of inflammation and necrosis (0-not present, 1-minimal, 2-mild, 3-moderate, 4-severe). In addition, the inflammatory cell constituents were characterized for each subject.
Results
Polysorbate 20 (PS20) and polysorbate 80 (PS80) inhibit O104:H4 biofilm formation
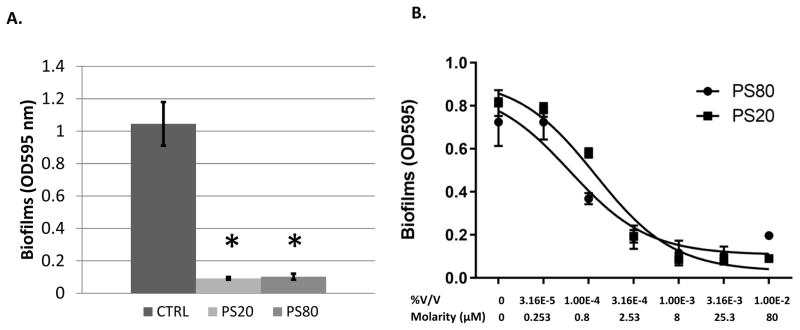
Twenty one anti-biofilm compounds were assayed that were either described in the published literature or were being developed in the authors’ laboratory for their ability to inhibit O104:H4 biofilm formation in a standard CV microtiter biofilm assay (Table 1). Nineteen compounds that exhibit anti-biofilm activity against other bacteria exhibited no significant effect on O104:H4. However, the nonionic surfactants PS80 and PS20, significantly reduced O104:H4 biofilm formation at concentrations of 0.01% (Fig. 1A). Polysorbates were previously shown to inhibit biofilm formation in Pseudomonas aeruginosa and other pathogenic bacteria, including six clinical E. coli isolates (Toutain-Kidd et al. 2009). The nature of these E. coli was not described, making this work the first report of polysorbate inhibition of O104:H4 biofilm formation. The in vitro efficacy of these compounds was determined by measuring O104:H4 biofilm formation at doses ranging from 0.01% to 0.0000316% and it was shown that both PS20 and PS80 have low micromolar effective concentrations of 50% inhibition (EC50) at 0.00006% (0.54 μM) and 0.0001% (0.81 μM), respectively (Fig. 1B). Polysorbates did not inhibit growth of O104:H4 at any concentration examined (Supplementary material Fig. S1).
Table 1.
Anti-biofilm compounds examined in this study.
| Compound | References |
|---|---|
| ABC-1* | 19 |
| ABC-1 Derivatives* 6,8,14,17,19,21,27,31,34,52,53,54,62 | Unpublished |
| Diguanylate cyclase inhibitors* 3, 10, 18 | 20 |
| Dispersin B* | 9 |
| Norspermidine* | 11 |
| D-Tyrosine* | 12 |
| Polysorbate 20, polysorbate 80 | 24 |
Compounds which did not exhibit a significant reduction in O104:H4 biofilm formation (data not shown).
Figure 1.
PS80 inhibits biofilm formation. A. Anti-biofilm activity was assessed for PS80 and PS20 at 0.01% V/V. B. A dose response curve determined EC50 values of 0.00006% (0.54 μM) for PS20 and 0.0001% (0.81 μM) for PS80. The EC50 curve was fit using PRISM (Graphpad) with a log-dose vs response, three parameters, nonlinear regression. * Indicates P < 0.05 based on students paired t-test. The error bars represent the SD (n=3).
PS20 and PS80 reduce initial attachment and disperse pre-formed biofilms
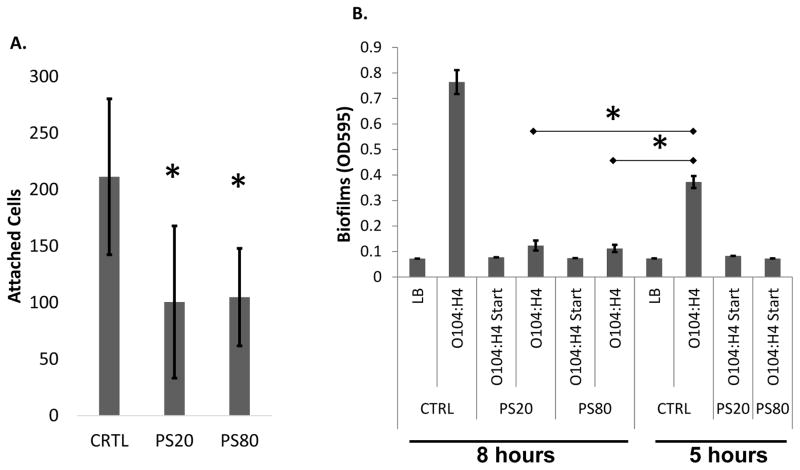
It was hypothesized that the polysorbates might negatively impact biofilms by blocking initial attachment to surfaces, the earliest process of biofilm formation. O104:H4 was incubated with the polystyrene microtiter plates for 1 h in the presence and absence of PS20 and PS80 and attachment measured by quantifying attached cells stained with Syto 9 (Invitrogen). Total cells were counted in 3 fields of view chosen randomly from 6 wells. The hypothesis was supported as both PS20 and PS80 significantly reduced attached cells by one half (Fig. 2A).
Figure 2.
PS80 disperses biofilms and inhibits attachment. A. PS20 and PS80 at 0.01 % significantly reduced initial attachment. B. Biofilm dispersal was assayed with treatment of 0.01% PS20 or PS80. Biofilms were formed for 5 h then treated for an additional 3 h. O104:H4 Start indicates samples that were treated from time 0, and CTRL indicates addition of the vehicle dH2O. * Indicates P < 0.05 based on students paired T test. The error bars represent the SD (n=3).
To determine whether PS80 can disperse a preformed biofilm, biofilms were grown in microtiter plates for 5 h then a subset of these biofilms were exposed to 0.01% PS20, 0.01% PS80, or the vehicle control. Biofilms were allowed to grow for an additional 3 h. A control group was subjected to treatment with polysorbates at time zero, and a duplicate plate was used to measure biofilm accumulation at 5 h. It was found that treatment of PS20 and PS80 at 5 h was able to disperse biofilms, leading to a significant inhibition of biofilms compared to both the 5 and 8 h untreated controls (Fig. 2B). Indeed, treatment with PS20 and PS80 at 5 h was nearly as effective at reducing biofilms as treatment with polysorbates at the initiation of the experiment. These results show that PS20 and PS80 can impact both initial biofilm formation and elicit dispersal of preformed biofilms.
Nonionic surfactants inhibit biofilms
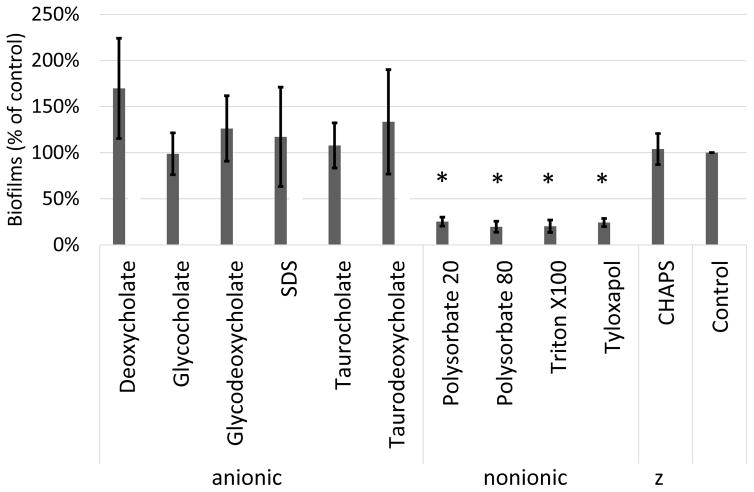
Polysorbates are nonionic surfactants that do not possess a charged head group and the authors considered whether all surfactants could inhibit O104:H4 biofilm formation. To explore this question, the activity of other surfactants to inhibit O104:H4 biofilms was tested. Six anionic surfactants, two additional nonionic surfactants, and a zwitterion surfactant were analyzed for O104:H4 biofilm formation. The nonionic surfactants Triton X-100 and tyloxapol also reduced O104:H4 biofilms similar to PS20 and PS80. However, neither the zwitterion nor the anionic surfactants possessed anti-biofilm activity (Fig 3). In fact, some of the anionic surfactants tested, which contain components of human bile, increased O104:H4 biofilms, although this increase was not statistically significant.
Figure 3.
Nonionic surfactants inhibit biofilms. A variety of surfactants were measured for biofilm inhibition with PS80 concentrations of 0.01% V/V. “z” indicates zwitterion. The error bars represent the SD (n=3). * Indicates P < 0.05 based on students paired T test compared with the control condition.
PS80 does not reduce colonization of O104:H4 in a mouse infection model
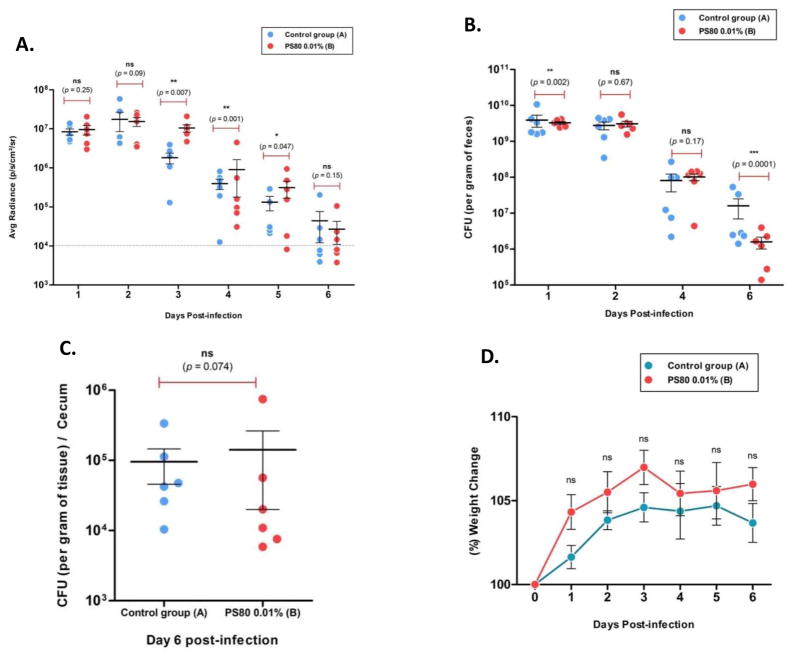
It has previously been hypothesized that in vivo biofilm formation correlates with virulence factor expression (Al Safadi et al. 2012). Thus, the impact of PS80 upon infection of mice with bioluminescent O104:H4 was determined. For this experiment, PS80 was added to the drinking water of O104:H4 infected mice at a concentration of 0.01%, and this group was compared to an untreated infected control group. As PS20 and PS80 exhibit almost identical anti-biofilm properties in vitro, only examine PS80 was chosen to examine in vivo. Mice were then monitored over a 6-day period using the In Vivo Imaging System (IVIS) for the number of viable bacteria in the intestinal tract. Additionally, viable O104:H4 in feces were quantified by selective plating on LB agar containing streptomycin at 100 μg ml−1 and kanamycin at 50 μg ml−1 which selects for O104:H4 strain RJC001. It was observed that there was no significant reduction in luminescence in the PS80 treated group of mice, suggesting that PS80 did not impact bacterial colonization (Fig. 4A). The PS80 treated mice in days 3, 4, and 5 had modest but significant increases in luminescence. Similarly, bacteria recovered in feces did not show a significant reduction at day 2 or day 4 and a significant but marginal reduction at day 1 (Fig. 4B). At day 6, the fecal bacteria load was significantly lower in the treated group; however, this was not supported by equivalent in vivo luminescence at day 6 (Fig. 4B). At the end of 6 days the mice were euthanized and tissue samples were collected from the cecum. Viable O104:H4 was quantified in the cecum and there was no significant difference between the treated and untreated mice (Fig. 4C). One possible interpretation of the discrepancy between fecal and cecal counts is that O104:H4 at a lower density in the presence of PS80 survives less well in the large intestine. The weight of the mice was also measured and normalized to the starting weight. The PS80 treated mice gained weight faster and had higher weight gains than the untreated mice during every day of the experiment, but this difference was not statistically significant (Fig. 4D).
Figure 4.
PS80 does not reduce O104:H4 colonization. A. The number of colonizing bacteria was measured daily for six days by average radiance using the IVIS. B. The number of O104:H4 CFU in feces was quantified by serial dilution plating on selective media. C. At day 6, viable O104:H4 were quantified from harvested cecum by dilution plating. D. Mouse weight during the infection was measured daily and normalized by percentage of the starting weight. (n=6)
PS80 inhibits virulence of O104:H4 in a mouse infection model
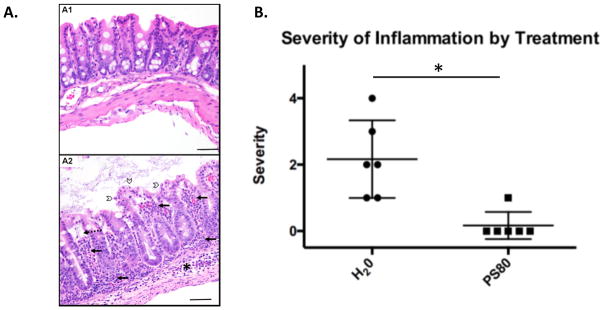
Although colonization was not significantly affected by PS80, the pathology of the cecum was quantified to determine if there was a difference in severity of disease. Cecum samples were fixed and immobilized in paraffin and thin sections were mounted on slides. followed by staining with hematoxylin and eosin. These sections were scored blindly for tissue pathology. All animals in the untreated group exhibited inflammation ranging from mild to severe, and in 5/6 animals the inflammation was neutrophilic (Table 2, Fig. 5A). By comparison, only 1/6 animals in the PS80 treated group exhibited any inflammation, which was scored as mild and was not neutrophilic (Table 2, Fig. 5A). Moreover, the only two animals that presented tissue necrosis were from the untreated group (A2 and A6), and these two animals exhibited the most severe inflammation, with the severity of inflammation proportional to the severity of necrosis (Table 2). Additional histopathologic findings in the untreated group included dilation of lamina propria lymphatic vessels and submucosal edema in animals with inflammation. The difference of the average in inflammation score between the two treatment groups was statistically significant (Fig 5B). These results indicate that treatment with PS80 did not significantly impact colonization levels but did significantly reduce the pathology of infecting O104:H4.
Table 2.
Histopathology scores.
| Animal ID | Inflammation* | Necrosis | Treatment |
|---|---|---|---|
| A1 | 2 (M>N) | - | None |
| A2 | 3 (N=M) | + (Individual cells) | None |
| A3 | 2 (N=M) | - | None |
| A4 | 1 (M>N) | - | None |
| A5 | 1 (M) | - | None |
| A6 | 4(N>M) | +++ (Erosion and ulceration) | None |
| B1 | 0 | - | PS80 |
| B2 | 1(M) | - | PS80 |
| B3 | 0 | - | PS80 |
| B4 | 0 | - | PS80 |
| B5 | 0 | - | PS80 |
| B6 | 0 | - | PS80 |
M=mononuclear; N=neutrophil.
Figure 5.
PS80 prevents O104:H4 pathology. A. Hematoxylin and eosin stained slides that are representative of the group are shown. A1 shows a PS80 treated animal with normal serosa, muscularis, submucosa, lamina propria and epithelium (from bottom to top). A2 shows an untreated (H20 group) animal with neutrophilic and mononuclear inflammation of the submucosa (*), and lamina propria (solid arrows), dilation of lymphatic vessels (dashed arrow), and epithelial cell necrosis and sloughing (open arrowheads). B. The mean inflammation scores from cecum of mice sacrificed at day 6 as measured by histopathology is shown. * Indicates P < 0.05 based on students paired T test. The error bars represent the SD (n=6).
Discussion
The E. coli O104:H4 strain that led to the devastating outbreak across Europe in 2011 resulted from the evolution of a novel bacterial pathogen that combined the superior colonization and persistence abilities of EAEC with high toxin production from an O157:H7 stx phage. This outbreak led to the highest prevalence of HUS ever recorded. Studies utilizing a germ-free mouse model suggested that in vivo biofilm formation was correlated with high levels of virulence factor expression, including toxin gene expression (Al Safadi et al. 2012). It was hypothesized that this was a causative duality in that the high-cell density state of a biofilm induced toxin gene expression, and a disruption of in vivo biofilm formation would reduce disease severity. To test this hypothesis, searches were made for compounds that disrupted biofilm formation by O104:H4 to determine if they impact in vivo disease outcomes. The results indicated that O104:H4 was highly resistant to the majority of anti-biofilm compounds that were examined as they had no significant impact on in vitro biofilm formation. However, the surfactants PS20 and PS80 were determined to be potent O104:H4 anti-biofilm compounds, both inhibiting the formation of biofilms and dispersing preformed biofilms at sub-micromolar EC50 concentrations. In support of the hypothesis, treating O104:H4 infected mice with PS80 completely abolished clinical symptoms.
The mechanism of PS80 inhibition of biofilms remains to be determined, although the present results offer some clues. PS20 and PS80 appeared to function at all levels of biofilm formation, impacting both initial adherence and dispersing mature biofilms. Thus, it was hypothesized that the target(s) of PS20 and PS80 are essential for all stages of O104:H4 biofilm formation. Of note, other nonionic surfactants such as Triton X100 and Tyloxapol also disrupted biofilms, but anionic and zwitterion surfactants had no effect. As the nonionic surfactants do not share significant atomic compositions, this result suggests that it is the physical properties of these molecules, rather than their specific chemical moieties, that inhibits O104:H4 biofilms. The matrix material of biofilms is generally anionic, and thus cationic surfactants have been shown to disperse biofilms. An example of this is the surfactant delmopinol, which is used to disrupt oral biofilms (Santos et. al. 2010).
The molecular mechanisms by which O104:H4 forms biofilms has not yet been determined. Cyclic di-GMP signaling has been described to promote biofilms of O104:H4 by inducing curli fibers, and it was hypothesized that the curli led to inflammation increasing the ability of toxin to access the bloodstream (Richter et al. 2014). Interestingly, PS20 was recently shown to disrupt UPEC pellicle formation by inhibiting the ability of curli fibers to form an extracellular network (Wu et al. 2013). The authors are currently exploring whether nonionic surfactants disrupt O104:H4 biofilms by inhibiting curli function.
Treatment of infected mice with PS80 had no significant impact on in vivo colonization even though this compound was a potent inhibitor of biofilm formation and able to disperse preformed biofilms in vitro. Rather, major differences were seen in the pathological outcomes of colonization including virtually no tissue inflammation or necrosis in treated animals upon a blinded histopathological scoring of infected tissue. Of note, this reduction in pathology occurred even during days in which treated mice exhibited increased colonization as measured by in vivo luminescence. This increased luminescence may be due to dispersal from biofilms as bacteria in biofilms may be oxygen limited which is a requirement for bioluminescence.
The mechanism by which PS80 inhibits O104:H4 pathology remains to be determined although the in vitro results presented here and in other studies would suggest that inhibition of biofilm formation is responsible. The severity of pathogenic E. coli infections derives from high levels of Shiga toxin entering the bloodstream resulting in HUS. These results are consistent with a prior study that found the pAA plasmid promotes increased biofilm formation and translocation of Stx2 across an in vitro epithelial cell monolayer (Boisen et al. 2014). The inhibition of biofilm formation may decrease disease severity by reducing Stx2 expression and/or decreasing Stx2 translocation. In this study, the analysis of Stx2 production from the cecal contents of mice harvested at day 6 was unable to detect stx2 gene expression using quantitative RT-PCR. In the mouse model used here, the infection was nearly resolved by day 6 exhibiting a 3 to 4 log10 decrease in viable bacteria (Fig. 4B), which was likely responsible for the inability to detect in vivo stx2 gene expression. Alternatively, PS80 treatment could function by modulating the immune response to O104:H4 or alter its ability to adhere to epithelial cells, although contrary to its known anti-biofilm activity, there appears to be no published literature to support such activity for PS80.
Regardless of the mechanism, these studies suggest that polysorbate administration is an attractive approach to alter an O104:H4 infection from a severe life threatening condition to a self-limiting mild enteritis. PS20 and PS80 classify as O104:H4 anti-infective compounds. Unlike traditional antibiotics, these compounds do not significantly impact growth, but rather they modulate an important virulence property (Sintim et al. 2010). Importantly, PS80 reduces virulence with the need for additional antimicrobials. Anti-infective approaches are an attractive strategy to treat infectious disease as they convert dangerous pathogens into more mild infections that the body can naturally resolve. In addition, it is hypothesized that the selection for resistance to such compounds would be much lower than antibiotics (Sintim et al. 2010). Indeed, PS20 and PS80 treatment of O104:H4 would exhibit virtually no selection for resistance as these compounds do not significantly impact gut colonization. An additional advantage of these compounds is that they should be much less disruptive to the normal gut microbiota than traditional antibiotics as polysorbates are common, non-toxic food additives that gut microbiota frequently encounter. Maintaining an intact gut microbiome should be beneficial in resolving O104:H4 infections and prevent other secondary enteric infections that rely on microbiota disruption such as Clostridium difficile (Kelly and LaMont 1998).
The present research shows that polysorbates are potent anti-biofilm compounds of O104:H4 both in vitro and in vivo. PS20 and PS80 have been designated Generally Recognized as Safe (GRAS) compounds by the FDA and are thus common components of food. Ice creams can contain 0.02% or higher PS80, a concentration that is orders of magnitude higher than the in vitro EC50 to inhibit the O104:H4 biofilms determined here (Muse and Hartel 2004). Interestingly, non-symptomatic O104:H4 infections were reported during the 2011 outbreak (Balabanova et al. 2013), and it is intriguing to speculate that one factor that may have contributed to these cases is consumption of polysorbates in food products. Importantly, no data yet exist to support this speculation, but given the amount of data collected on the diets of infected individuals this hypothesis could be examined. Pathogenic E. coli often cause disease by contaminating food products, and the 2011 O104:H4 epidemic was mediated by colonized fenugreek sprouts (Buchholz et al. 2011). Due to the safety of polysorbates and their ability to disperse in vitro biofilms, washes of contaminated food with polysorbates could reduce infection through colonized food. PS80 administration in the drinking water of mice prevented nearly all histopathology. If this result is confirmed in additional animal studies and is representative of human infections, PS80 could be effective at significantly reducing HUS occurrence in future O104:H4 outbreaks.
Supplementary Material
Polysorbates do not inhibit growth of E. coli O104:H4. Growth of O104:H4 in LB medium was monitored over time in the presence of 0.01 % PS20, PS80, or 50 μg ml−1 kanamycin and tetracycline 1 μg ml−1 (kill) as a positive control. The error bars represent the SD (n=3).
Acknowledgments
The authors are grateful to Robert Parker and Shannon Manning for efforts to quantify in vivo stx expression and strain TW16133.
Funding Information
This work was supported by grants NIH grants AI079154 to AGT, and AI090872, GM110444, GM109259, and NSF grant MCB1253684 to C.M.W.
References
- Al Safadi R, Abu-Ali GS, Sloup RE, Rudrik JT, Waters CM, Eaton KA, Manning SD. Correlation between in vivo biofilm formation and virulence gene expression in Escherichia coli O104:H4. PLoS ONE. 2012;7:e41628. doi: 10.1371/journal.pone.0041628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanova Y, Klar S, Delere Y, Wilking H, Faber MS, Lassen SG, Gilsdorf A, Dupke S, Nitschke M, Sayk F, et al. Serological evidence of asymptomatic infections during Escherichia coli O104:H4 outbreak in Germany in 2011. Plos One. 2013;8:e73052. doi: 10.1371/journal.pone.0073052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutin L, Martin A. Outbreak of Shiga toxin-producing Escherichia coli (STEC) O104:H4 infection in Germany causes a paradigm shift with regard to human pathogenicity of STEC strains. J Food Prot. 2012;75:408–418. doi: 10.4315/0362-028X.JFP-11-452. [DOI] [PubMed] [Google Scholar]
- Bielaszewska M, Idelevich EA, Zhang W, Bauwens A, Schaumburg F, Mellmann A, Peters G, Karch H. Epidemic Escherichia coli O104:H4: effects of antibiotics on Shiga toxin 2 production and bacteriophage induction. Antimicrob Agents Chemother. 2012;56:3277–82. doi: 10.1128/AAC.06315-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielaszewska M, Mellmann A, Zhang W, Kock R, Fruth A, Bauwens A, Peters G, Karch H. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect Dis. 2011;11:671–676. doi: 10.1016/S1473-3099(11)70165-7. [DOI] [PubMed] [Google Scholar]
- Boisen N, Hansen AM, Melton-Celsa AR, Zangari T, Mortensen NP, Kaper JB, O’Brien AD, Nataro JP. The presence of the pAA plasmid in the German O104:H4 Shiga toxin type 2a (Stx2a)-producing enteroaggregative Escherichia coli strain promotes the translocation of Stx2a across an epithelial cell monolayer. J Infect Dis. 2014;210:1909–1919. doi: 10.1093/infdis/jiu399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz U, Bernard H, Werber D, Bohmer MM, Remschmidt C, Wilking H, Delere Y, an der Heiden M, Adlhoch C, Dreesman J, et al. German outbreak of Escherichia coli O104:H4 associated with sprouts. N Engl J Med. 2011;365:1763–1770. doi: 10.1056/NEJMoa1106482. [DOI] [PubMed] [Google Scholar]
- Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, Bernard H, Fruth A, Prager R, Spode A, et al. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med. 2011;365:1771–1780. doi: 10.1056/NEJMoa1106483. [DOI] [PubMed] [Google Scholar]
- Kaplan JB, Velliyagounder K, Ragunath C, Rohde H, Mack D, Knobloch JK, Ramasubbu N. Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms. J Bacteriol. 2004;186:8213–8220. doi: 10.1128/JB.186.24.8213-8220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CP, LaMont JT. Clostridium difficile infection. Annu Rev Med. 1998;49:375–390. doi: 10.1146/annurev.med.49.1.375. [DOI] [PubMed] [Google Scholar]
- Kolodkin-Gal I, Cao S, Chai L, Bottcher T, Kolter R, Clardy J, Losick R. A self-produced trigger for biofilm disassembly that targets exopolysaccharide. Cell. 2012;149:684–692. doi: 10.1016/j.cell.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. D-amino acids trigger biofilm disassembly. Science. 2010;328:627–629. doi: 10.1126/science.1188628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellmann A, Harmsen D, Cummings CA, Zentz EB, Leopold SR, Rico A, Prior K, Szczepanowski R, Ji Y, Zhang W, et al. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS ONE. 2011;6:e22751. doi: 10.1371/journal.pone.0022751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munera D, Ritchie JM, Hatzios SK, Bronson R, Fang G, Schadt EE, Davis BM, Waldor MK. Autotransporters but not pAA are critical for rabbit colonization by Shiga toxin-producing Escherichia coli O104:H4. Nat Commun. 2014;5:3080. doi: 10.1038/ncomms4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muse MR, Hartel RW. Ice cream structural elements that affect melting rate and hardness. J Dairy Sci. 2004;87:1–10. doi: 10.3168/jds.S0022-0302(04)73135-5. [DOI] [PubMed] [Google Scholar]
- Rahal EA, Fadlallah SM, Nassar FJ, Kazzi N, Matar GM. Approaches to treatment of emerging Shiga toxin-producing Escherichia coli infections highlighting the O104:H4 serotype. Front Cell Infect Microbiol. 2015;5:24. doi: 10.3389/fcimb.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter AM, Povolotsky TL, Wieler LH, Hengge R. Cyclic-di-GMP signalling and biofilm-related properties of the Shiga toxin-producing 2011 German outbreak Escherichia coli O104:H4. EMBO Mol Med. 2014;6:1622–1637. doi: 10.15252/emmm.201404309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross BN, Rojas-Lopez M, Cieza RJ, McWilliams BD, Torres AG. The role of long polar fimbriae in Escherichia coli O104:H4 adhesion and colonization. PLoS One. 2015;10:e0141845. doi: 10.1371/journal.pone.0141845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambanthamoorthy K, Gokhale AA, Lao W, Parashar V, Neiditch MB, Semmelhack MF, Lee I, Waters CM. Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner. Antimicrob Agents Chemother. 2011;55:4369–4378. doi: 10.1128/AAC.00583-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambanthamoorthy K, Parashar V, Smith JM, Kim E, Sloup RE, Semmelhack MF, Neiditch MB, Waters CM. Identification of small molecules that antagonize diguanylate cyclase enzymes to inhibit biofilm formation. Antimicrob Agents Chemother. 2012;56:5202–5211. doi: 10.1128/AAC.01396-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos O, Lindh L, Halthur T, Arnebrant T. Adsorption from saliva to silica and hydroxyapatite surfaces and elution of salivary films by SDS and delmopinol. Biofouling. 2010;26:697–710. doi: 10.1080/08927014.2010.506609. [DOI] [PubMed] [Google Scholar]
- Sintim HO, Smith JA, Wang J, Nakayama S, Yan L. Paradigm shift in discovering next-generation anti-infective agents: targeting quorum sensing, c-di-GMP signaling and biofilm formation in bacteria with small molecules. Future Med Chem. 2010;2:1005–1035. doi: 10.4155/fmc.10.185. [DOI] [PubMed] [Google Scholar]
- Steiner TS. The worst of both worlds: examining the hypervirulence of the shigatoxigenic/enteroaggregative Escherichia coli O104:H4. J Infect Dis. 2014;210:1860–1862. doi: 10.1093/infdis/jiu400. [DOI] [PubMed] [Google Scholar]
- Torres AG, Cieza RJ, Rojas-Lopez M, Blumentritt CA, Souza CS, Johnston RK, Strockbine N, Kaper JB, Sbrana E, Popov VL. In vivo bioluminescence imaging of Escherichia coli O104:H4 and role of aerobactin during colonization of a mouse model of infection. BMC Microbiol. 2012;12:112. doi: 10.1186/1471-2180-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutain-Kidd CM, Kadivar SC, Bramante CT, Bobin SA, Zegans ME. Polysorbate 80 inhibition of Pseudomonas aeruginosa biofilm formation and its cleavage by the secreted lipase LipA. Antimicrob Agents Chemother. 2009;53:136–145. doi: 10.1128/AAC.00500-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Lim JY, Fuller GG, Cegelski L. Disruption of Escherichia coli amyloid-integrated biofilm formation at the air-liquid interface by a polysorbate surfactant. Langmuir. 2013;29:920–926. doi: 10.1021/la304710k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangari T, Melton-Celsa AR, Panda A, Boisen N, Smith MA, Tatarov I, De Tolla LJ, Nataro JP, O’Brien AD. Virulence of the Shiga toxin type 2-expressing Escherichia coli O104:H4 German outbreak isolate in two animal models. Infect Immun. 2013;81:1562–1574. doi: 10.1128/IAI.01310-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Bielaszewska M, Kunsmann L, Mellmann A, Bauwens A, Kock R, Kossow A, Anders A, Gatermann S, Karch H. Lability of the pAA virulence plasmid in O104:H4: implications for virulence in humans. Plos One. 2013;8:e66717. doi: 10.1371/journal.pone.0066717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Polysorbates do not inhibit growth of E. coli O104:H4. Growth of O104:H4 in LB medium was monitored over time in the presence of 0.01 % PS20, PS80, or 50 μg ml−1 kanamycin and tetracycline 1 μg ml−1 (kill) as a positive control. The error bars represent the SD (n=3).