Figure 4.
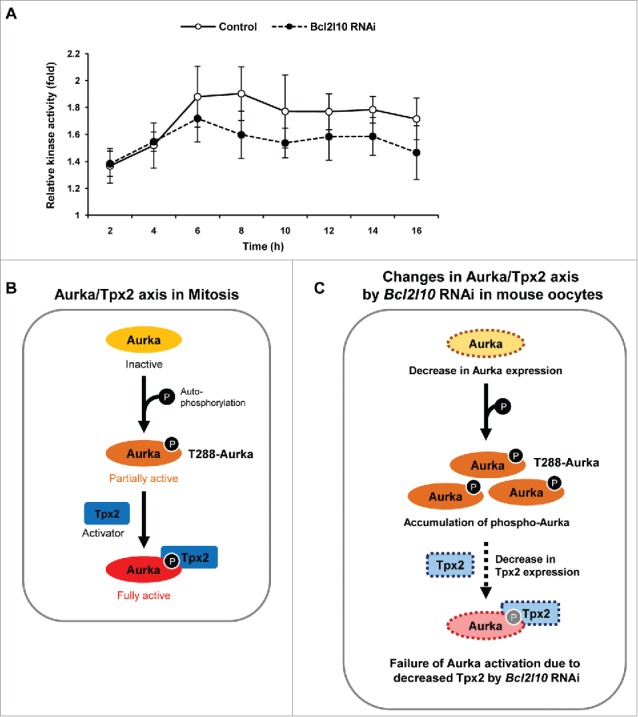
Bcl2l10 RNAi reduces the catalytic activity of Aurka. (A) Aurka activity was assessed by measuring the phosphorylation of Histone H3, an Aurka substrate. Oocytes were collected every 2 h during in vitro maturation. Kinase activity was determined by quantifying the phosphorylation of substrates relative to that of control oocytes at 0 h. The Y-axis depicts the fold changes (log scale) and the X-axis depicts the time points at which oocytes were sampled during oocyte in vitro maturation. Five oocytes were used per time point. Error bars represent SD (B) Schematic diagram for the molecular mechanism of Aurka activation known in mitosis. First, Aurka was partially activated by phosphorylation at threonine 288 in its activation loop. Second, partially activated T288-Aurka was fully activated by the binding of the activator protein Tpx2. Binding with Tpx2 protects phosphorylated Aurka from dephosphorylation by protein phosphatase 1. (C) Schematic diagram depicting the speculated roles of Bcl2l10 in the Aurka/Tpx2 axis in the mouse oocytes based on the results of Bcl2l10 RNAi treatment in the present study. When Bcl2l10 was absent, Aurka and Tpx2 expression decreased, whereas the amount of T288-Aurka [auto-phosphorylated Aurka] increased. We propose that Aurka activity decreased because partially active T288-Aurka could not be fully activated due to decreased Tpx2. Therefore, we concluded that the down-regulation of Tpx2 is a decisive factor for the full activation of Aurka.
