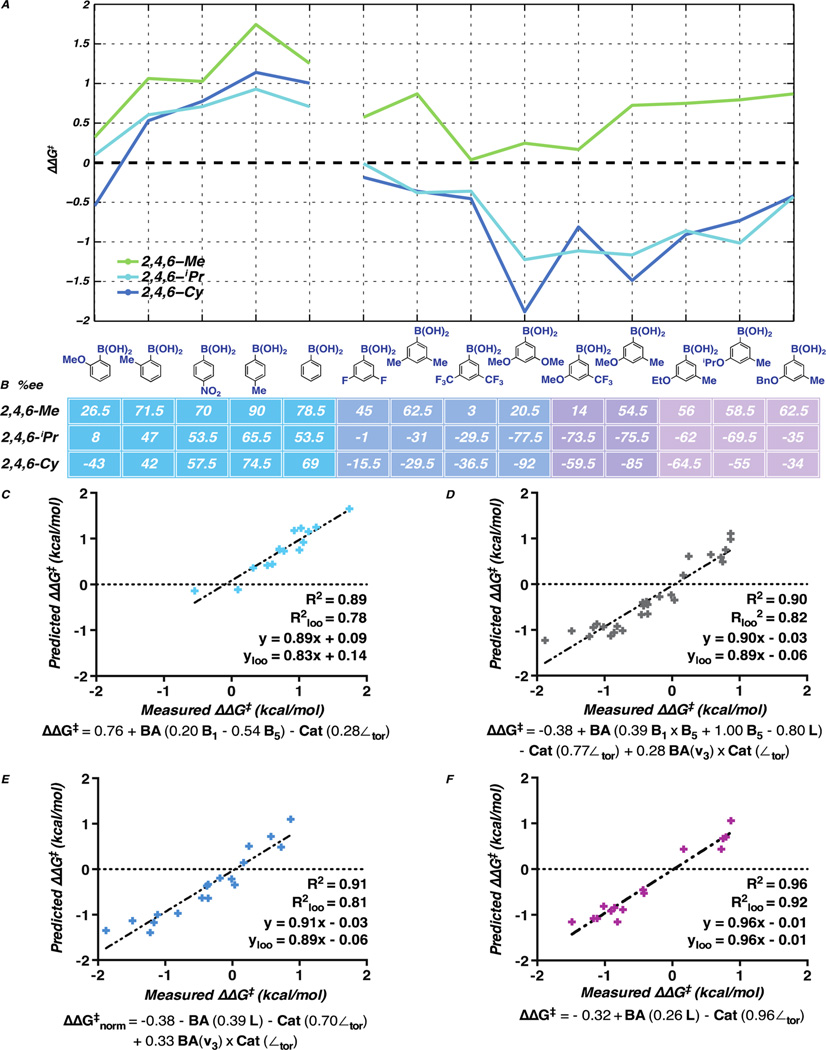
Figure 9.
(A) Graphical representation of catalyst structure-selectivity trends as a function of BA structure. (B) Enantioselectivity of 2 using catalysts bearing 2,4,6-trisubstituted aryl substituents with various BAs. (C–F) Mathematical correlation of normalized catalyst and BA molecular descriptors to enantioselectivity (ΔΔG‡) for 2- and 4-substituted aryl BAs (C), all 3,5-disubstituted aryl BAs (D), 3,5-disubstituted aryl BAs with symmetrical substitution or a methoxy substituent (E), and 3-alkoxy-5-methyl substituted aryl BAs (F). All reactions were conducted on 0.05 mmol scales with respect to allylic alcohol 1.