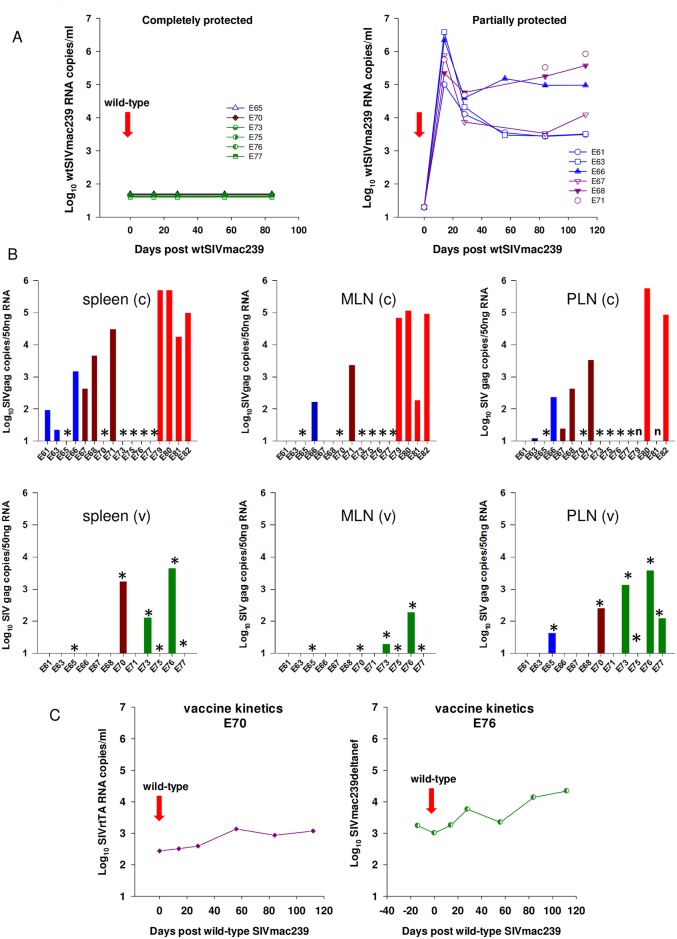
Fig 3. Virus–specific vRNA levels and vaccine sequestration.
(A). Virus-specific plasma vRNA levels determined by real-time PCR in the period post wild-type SIVmac239 challenge (red arrows). Levels of wild-type-specific plasma viral RNA quantitatively assessed with SIVmac239-specific vRNA assays separated vaccinates into completely protected (n = 6; E65, E70, E73, E75, E76, E77) or partially protected (n = 6; E61, E63, E66, E67, E68, E71) groups. (B). Cell-associated RNA (CA-RNA) expressed as viral RNA copies/50ng RNA normalised to GAPDH for either challenge (c) or vaccine (v) viruses for spleen, mesenteric lymph nodes (MLN) and peripheral lymph nodes (PLN), represented by initial colour coded group (ie Group A, blue; Group B; dark red; Group C, green, Group D, bright red). Asterisks (*) identify completely protected macaques as represented in panel A. E75 (SIVmac239Δnef) was qualitatively positive only in PLN, signalling at the limit of assay detection. n indicates no sample available for testing. (C). Vaccine virus replication profiles for SIVrtTA vaccinate E70 and SIVmac239Δnef vaccinate E76 are shown, exhibiting sustained levels of vaccine replication post-wtSIVmac239 challenge (red arrows).