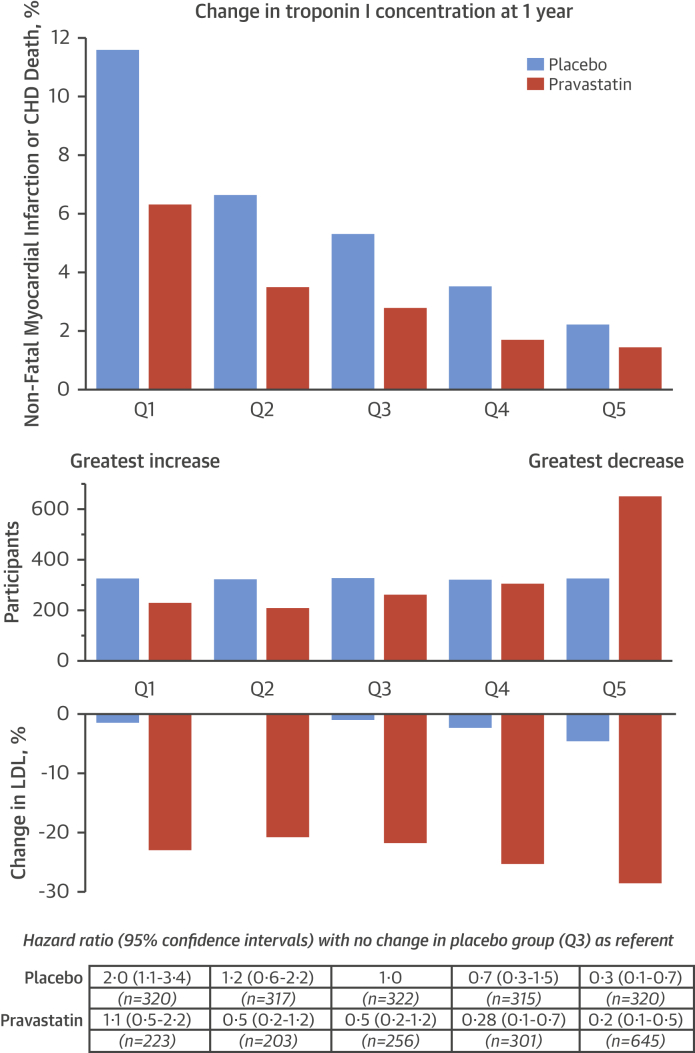
Figure 3.
Change in Troponin Concentration at 1 Year Stratified by Treatment Allocation and Nonfatal Myocardial Infarction or Coronary Heart Disease Death at 5 Years
(Top) Nonfatal myocardial infarction or death from coronary heart disease (CHD) at 5 years in those taking pravastatin (orange) and placebo (blue) stratified into fifths by change in troponin concentration in the placebo group: Q1 = >26% increase; Q2 = 2% to 25% increase; Q3 = 2% increase to 13% decrease; Q4 = 13% to 27% decrease; Q5 = ≥27% decrease. (Middle) Twice as many participants were in the lowest-risk group (Q5), with >27% reductions in troponin concentration (645 vs. 320 on placebo; p < 0.001), and 30% fewer were in the highest-risk group (Q1) with >26% increase in troponin concentration (223 vs. 320 on placebo; p < 0.001). (Bottom) Using participants in the placebo group whose troponin concentrations were unchanged as a referent (Q3), hazard ratios for the primary outcome were determined for each fifth after adjustment for age, body mass index, heart rate, systolic blood pressure, diastolic blood pressure, high-density lipoprotein and low-density lipoprotein (LDL) cholesterol, symptoms of angina, diabetes, hypertension, family history of premature coronary heart disease, minor electrocardiographic abnormalities, nitrate use, and smoking status. The risk of the primary endpoint in participants taking pravastatin was 5-fold lower in those with the greatest reduction in troponin concentration (hazard ratio: 0.23; 95% confidence interval: 0.10 to 0.53) compared to those with the greatest increase in troponin concentration (hazard ratio: 1.08; 95% confidence interval: 0.53 to 2.21; p < 0.001 for trend) despite similar reductions in LDL cholesterol concentration (22% to 28%).