Figure 8. PurH has a selective beneficial effect on EcDHFR activity only (A) Catalytic rate (kcat), Michaelis coefficient (KM), and the catalytic efficiency (kcat/KM) of EcDHFR, DHFR6 and DHFR11 for dihydrofolic acid (DHF) in the presence of low concentrations of PurH.
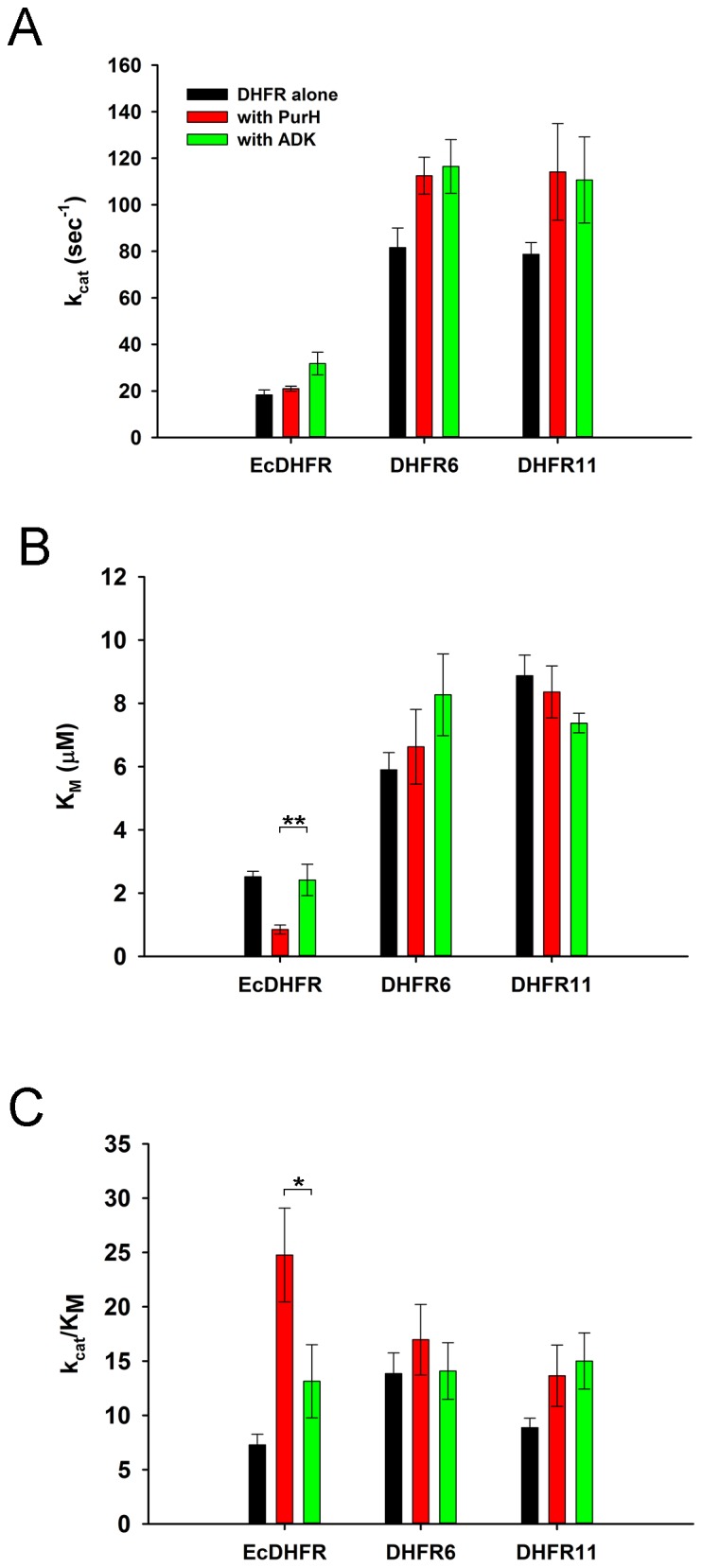
10 nM DHFR were pre-incubated with 15 nM of PurH or ADK and, subsequently, the initial rate of conversion of NADPH to NADP+ was measured at 340 nm. For determination of kcat and KM, the concentration of DHF was varied from 0.1 µM to 16 μM for EcDHFR and from 1 μM to 64 μM for DHFR6 and 11, while NADPH concentration was fixed at saturation (150 µM). Each data point is an average of 3–5 independent measurements and the error bars represent standard deviation. Both PurH and ADK resulted in an increase in kcat of all the DHFRs, however PurH caused a significant drop in KM and a concomitant increase in kcat/KM for EcDHFR only. In all panels, * indicates p-value<0.05, while ** indicates p-value<0.001. Therefore, at physiological ratios of proteins, PurH is only beneficial for EcDHFR. This observation suggests an evolutionary functional relationship between PurH and EcDHFR at physiological concentrations. Orthologous DHFRs that have diverged during the course of evolution no longer have this benefit from E. coli PurH.
