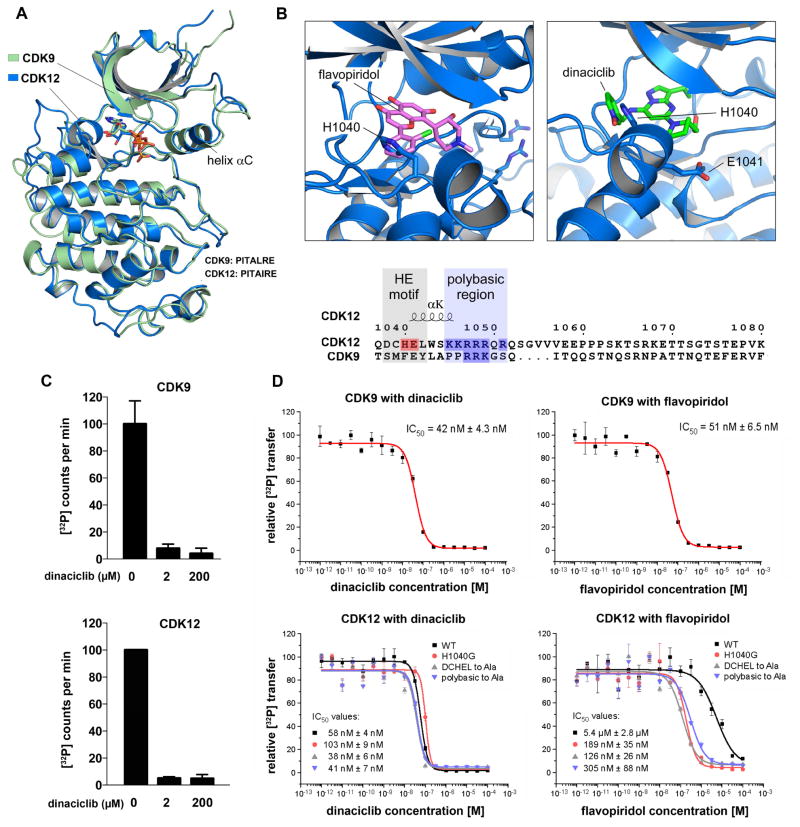
Figure 1. Dinaciclib is a potent inhibitor of CDK12 in addition to CDK9.
(A) Tertiary structural alignment of CDK9 and CDK12. (B) Sequence corresponding to the variance in the C-terminal extension helix of the kinase domains of CDK12 and CDK9 (see also Figure S1), as well as structural modeling of the orientations of flavopiridol and dinaciclib in relation to H1040 and E1041 of the CDK12 ATP binding site. The benzene ring of flavopiridol shows a steric clash with H1040 of Cdk12, whereas the pyridine-N-oxide ring of dinaciclib overlaps the aromatic H1040 side chain, resulting in a possible stacking of the aromatic ring systems that stabilizes the interaction and contributes to binding specificity. (C) In vitro kinase assays using pS7-CTD[3] as substrate and 0.2 μM cyclin T-CDK9 and cyclin K-CDK12 holoenzyme complexes alone or with and 10x or 1000x dinaciclib. (D) Concentration series of dinaciclib and flavopiridol for cyclin T1-CDK9 and cyclin K-CDK12 at 0.2 μM kinase concentration. The IC50 values against Cdk9 and CDK12 are comparable for dinaciclib but disparate for flavopiridol. Introduction of the indicated mutations sensitizes CDK12 to flavopiridol. All data are reported as the mean ± SD from three independent experiments.