Abstract
Background
SORL1 rs1699102 is associated with the risk of late-onset Alzheimer's disease (AD). However, the effects of this SNP on cognition and brain structure during normal aging are unclear. This study aims to examine the effects of the rs1699102 polymorphism on age-related cognitive decline and cortical gray matter reduction in Chinese Han population.
Methods
780 non-demented adults completed a battery of neuropsychological tests. High-resolution T1-weighted structural magnetic resonance imaging (MRI) data from 89 of these subjects were also collected using a Siemens Trio 3.0 Tesla scanner.
Results
The T allele carriers displayed an accelerated age-related change in episodic memory and processing speed tests relative to the CC genotype. A similar pattern was observed in the age-related gray matter volume (GMV) reduction of the right middle temporal pole. The GMV in this region was significantly positively correlated with the episodic memory scores.
Conclusions
The SORL1 gene rs1699102 polymorphism has been found to be associated with age-related cognitive decline and GMV reduction of the right middle temporal pole in older adults. These findings elucidate how the SORL1 variants shape the neural system to modulate age-related cognitive decline and support the hypothesis that SORL1 may represent a candidate gene for late-onset AD.
Keywords: SORL1, cognitive decline, gray matter volume, non-demented elderly, Chinese
1. Introduction
Genetic factors play important roles in the development of late-onset Alzheimer's disease (AD). The apolipoprotein E (ApoE) ε4 allele is the most prominent susceptibility gene for AD [1]. In recent years, many more genes have been implicated to increase the risk of AD and shown to negatively impact cognition to varying degrees at very early stage of AD and even in cognitively intact elders [2, 3].
Sorting protein-related receptor with A-type repeats (SorLA, also known as LR11), a member of the low-density lipoprotein receptor family of ApoE receptors, has been identified to modulate amyloid precursor protein (APP) processing and amyloid-β (Aβ) production [4, 5]. Polymorphisms in the neuronal sortilin-related receptor (SORL1) gene that encodes SorLA have been implicated in late-onset AD [4, 6]. Evidence from an autopsy study showed that a SORL1 haplotype in the 3’ gene region that consists of a single nucleotide polymorphism (SNP) rs1699102 is associated with poor receptor expression in the brain of AD patients [7]. Although the risk allele might not be consistent across the different ethnic groups [4], the SNP rs1699102 and haplotypes that encompass this SNP have been found to be associated with a risk for AD [4, 8, 9].
In addition to the reported AD risk, the SORL1 gene has been implicated in the relationship between its variants and cognitive abilities in non-demented subjects [10, 11]. Reynolds and colleagues found that several SORL1 SNPs were associated with cognitive change trajectories in older adults [12]. Conversely, Liu and colleagues failed to find any significant association between the rs1699102 polymorphism and cognitive function [13]. To our knowledge, the relationship of this SNP with age-related cognitive changes in older adults remains inconclusive.
Recent brain magnetic resonance imaging (MRI) studies have revealed that several SORL1 variants are associated with hippocampal atrophy, the microstructural integrity of the fronto-temporal white matter tracts and other AD-related neurodegenerative changes [14-16]. To date, only one study has reported a significant association between the haplotypes in the region of SNP rs1699102 and hippocampal atrophy, as well as general cerebral atrophy in AD patients and unaffected siblings [16]. The rs1699102 effects on the whole-brain and additional brain regions have yet to be reported in the literature. Such data on normal aging will elucidate the neural substrates underlying the rs1699102-related risk of developing late-onset AD.
In the present study, 780 cognitively normal subjects completed a battery of neuropsychological tests, and high-resolution structural MRI on 89 of these subjects were collected to examine the effects of the SORL1 gene rs1699102 polymorphism on age-related cognitive decline and cortical gray matter volume (GMV) reduction in a large cohort of non-demented elderly Han Chinese. A voxel-based morphometry (VBM) analysis was used to assess regional GMV. We hypothesized that the SNP rs1699102 might modulate an age-related reduction in the GMV.
2. Methods
2.1 Participants and neuropsychological measures
This study included 780 native Chinese subjects from the BABRI (Beijing Aging Brain Rejuvenation Initiative) database. Details of participant selection have been previously described in our previous paper [17]. Participants were included in this study if they met the following criteria: (1) aged older than 50 years; (2) had received 6 years or more of education; (3) a score of no less than 24 on the Mini-Mental-Status Examination-Chinese version (MMSE) [18]; (4) no history of taking psychoactive medications; (5) no large vessel disease, such as cortical or subcortical infarcts and watershed infarcts; (6) no history of addictions, neurologic or psychiatric diseases, or treatment that would impair cognitive function. Participants meeting the following criteria were excluded from the MRI study: (1) unable to fulfill the physical demands of the MR scanning; (2) history of neurologic, psychiatric, or systemic illnesses effecting cerebral function, including serious vascular diseases, head trauma, tumor, current depression, alcoholism, and epilepsy. Written informed consent was obtained from each participant. The study was approved by the Institutional Review Board of Beijing Normal University.
To evaluate the general mental status and other cognitive function, participants underwent a series of neuropsychological tests involving 5 cognitive domains. The general mental status was assessed with the MMSE. The episodic memory tests consisted of the Auditory Verbal Learning Test (AVLT) [19] and Recall component of Rey-Osterrieth Complex Figure Test (ROCF) [20]. The attention and processing speed tests consisted of the Symbol Digit Modalities Test (SDMT) [21] and Trail Making Test A (TMT-A) [22]. The visual-spatial tests consisted of the Copy component of ROCF and Clock-Drawing Test (CDT) [23]. The language ability tests consisted of the Boston Naming Test (BNT) [24] and Category Verbal Fluency Test (CVFT) [25]. The executive function was tested using the Trail Making Test B (TMT-B) [22] and Stroop Color-word Test (Stroop) [26]. The demographic information and neuropsychological characteristics based on the SORL1 genotypes are shown in Table 1.
Table 1.
Demographics and Neuropsychological Test Results of Subjects Based on SORL1 Genotypes
| SNP ID | rs1699102 | Main Group effect p-value* | Group × Age interaction p-value† | |
|---|---|---|---|---|
| CC (n=617) | CT/TT (n=163) | |||
| Age, y (SD) | 64.51(7.30) | 65.35(7.23) | 0.189 | -- |
| Gender, n (male/female) | 221/396 | 68/95 | 0.165 | -- |
| Education, y (SD) | 11.34(3.22) | 11.44(3.28) | 0.712 | -- |
| APOE ε4, n (carriers/noncarriers) | 106/511 | 19/144 | 0.087 | -- |
| General mental status | ||||
| MMSE (SD) | 27.94(1.62) | 27.84(1.64) | 0.454 | 0.417 |
| Episodic memory | ||||
| AVLT-delay recall (SD) | 5.84(2.53) | 5.47(2.66) | 0.164 | 0.026‡ |
| AVLT-total (SD) | 30.76(9.28) | 29.76(9.37) | 0.347 | 0.004‡ |
| ROCF Recall (SD) | 13.64(6.21) | 13.13(6.69) | 0.429 | 0.021‡ |
| Spatial processing | ||||
| ROCF Copy (SD) | 33.30(3.33) | 33.55(3.04) | 0.372 | 0.898 |
| CDT (SD) | 24.92(3.39) | 24.33(4.26) | 0.051 | 0.347 |
| Language | ||||
| CVFT (SD) | 45.07(8.50) | 46.38(8.74) | 0.056 | 0.098 |
| BNT (SD) | 23.25(3.54) | 23.69(3.86) | 0.188 | 0.980 |
| Attention and processing speed | ||||
| SDMT (SD) | 35.30(11.29) | 35.37(11.11) | 0.478 | 0.178 |
| TMT-A time, s (SD) | 58.35(20.20) | 56.34(22.36) | 0.156 | 0.015‡ |
| Executive function | ||||
| TMT-B time, s (SD) | 177.93(69.75) | 169.97(64.43) | 0.143 | 0.259 |
| Stroop time, s (SD) | 77.06(22.51) | 76.66(23.73) | 0.839 | 0.578 |
Abbreviations: MMSE, Mini-Mental State Examination; AVLT, Auditory Verbal Learning Test; ROCF, Rey-Osterrieth Complex Figure; CDT, Clock-Drawing Test; CVFT, Category Verbal Fluency Test; BNT, Boston Naming Test; SDMT, Symbol Digit Modalities Test; TMT, Trail Making Test; Stroop, Stroop Color-word Test; SD, standard deviation.“--” indicates no data available.
Comparisons between groups were performed by using the Pearson χ2 test for gender and APOE ε4 and a two-sample t-test for age and education. An analysis of covariance (ANCOVA) was used to determine the main group differences in the neuropsychological test results with age, gender, education and APOE ε4 as covariates.
GLM was used to determine the interaction between age and genotypes with age, group, group × age as predictor variables and gender, education, APOE ε4 as covariates.
p< 0.05.
2.2 SORL1 genotyping
Custom Taqman SNP Genotyping Assays (Applied Biosystems, Foster City, USA) were used to prescreen for the rs1699102 genotype in the participants. An additional two SNPs, rs429358 and rs7412, which jointly define the APOE ε2 (with haplotype of rs429358-rs7414: T-T), ε3 (T-C), and ε4 alleles (C-C), were also genotyped [27]. The sample success rate for all of SNPs was 100%, and the reproducibility of all genotyping was 100% according to a duplication analysis of at least 10% of the genotypes. The rs1699102 genotyping indicated 163 T allele carriers (including 11 TT carriers and 152 CT carriers) and 617 CC homozygotes.
2.3 MRI data acquisition
The MRI data were collected from 37 subjects with the CT/TT genotypes and 52 subjects with the CC genotype using a Siemens Trio 3.0 Tesla scanner at the Imaging Center for Brain Research, Beijing Normal University and included 3D T1-weighted MRI scans. The high-resolution T1-weighted structural images were obtained using magnetization-prepared rapid gradient-echo sequences with the following parameters: 176 sagittal slices, slice thickness = 1 mm, repetition time (TR) = 1900 ms, echo time (TE) = 3.44 ms, field of view (FOV) = 256 × 256 mm2, acquisition matrix = 256 × 256.
2.4 Structural image preprocessing
The individual structural images (3D T1-weighted anatomical images) of all subjects were obtained using the VBM8 toolbox of Statistic Parametric Mapping (SPM) (http://dbm.neuro.uni-jena.de/vbm/). The images were bias-corrected, tissue classified, and normalized to the MNI-space using nonlinear only transformations to compare the relative differences in the regional GMV [28].
The homogeneity of gray matter (GM) images was examined using the covariance structure of each image with all other images, which helped to identify outliers. As a result, seven extreme outliers that showed anatomical abnormalities or artifacts were identified and excluded. The remaining 82 images were employed in the subsequent analyses. The modulated GM images were smoothed with a Gaussian kernel of 8 mm FWHW. A general linear model (GLM) using the individual GM images with gender, education and APOE genotype as covariates was constructed to test the interaction between genotypes and age with SPM (P < 0.001, uncorrected). To correct for multiple comparisons, GM clusters with a false discovery rate (FDR) corrected P < 0.05 were considered significant. The GMV were extracted from the voxels in the whole cluster for the subsequent correlation analyses.
2.5 Statistical Analysis
The data were subjected to a Hardy-Weinberg equilibrium test using the Plink program [29]. The differences between the SNP rs1699102 T allele carriers and non-carriers were assessed with the Pearson χ2 test for gender and APOE ε4 status, and a two-sample t-test was used for age and education. The GLM was applied to determine the effect of the interaction between genotypes and age on neuropsychological tests with gender, education and APOE ε4 status as covariates. Sensitivity analyses were applied to confirm the stability of effect. Spearman partial correlation analyses were used to investigate the relationship between the significantly interactional GM regions and the neuropsychological scores after adjusting for the covariates, such as rs1699102 genotypes (only for the global partial correlation analysis), age, gender, education and APOE ε4 status. All statistical analyses were performed using the SAS 9.3 software (SAS Institute, Cary, NC). A P value <0.05 was considered significant.
3. Results
3.1 Demographics and neuropsychological test results
The SNP rs1699102 did not deviate from the Hardy-Weinberg equilibrium in the total sample (P> 0.05). Table 1 lists the demographics and neuropsychological test scores of subjects stratified by the SORL1 genotypes. The age, gender, education, or APOE ε4 status did not significantly differ between the two groups. Notably, AVLT-delay recall, AVLT-total, ROCF Recall and the TMT-A time showed a significant age × group interaction, and sensitivity analyses in which the 11 TT participants were excluded from the GLMs did not change the results. Specifically, the T allele carriers displayed a faster age-related change (decrease in AVLT-delay recall, AVLT-total and ROCF Recall, increase in TMT-A time) in the scores relative to the CC genotype. The analysis of the main group effect failed to produce remarkable results.
3.2 The interactive effects between rs1699102 variants and age on the GMV
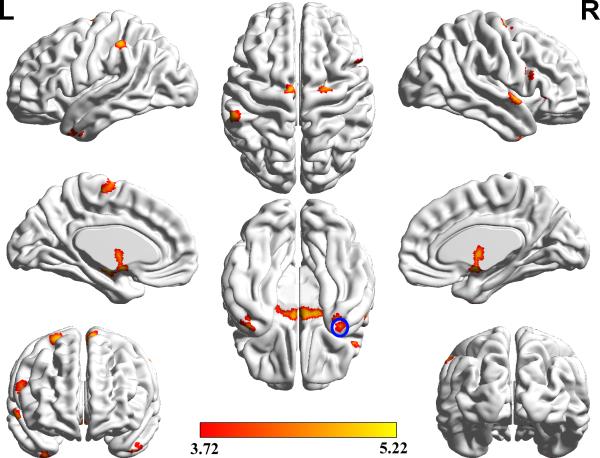
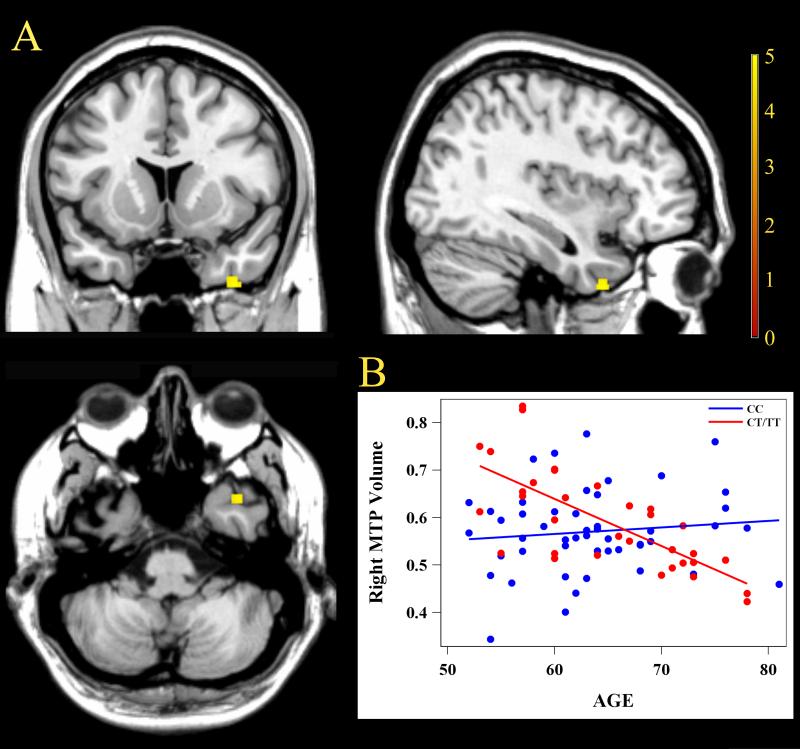
We examined the interaction of the GMV between age and groups in a sub-sample that included 33 subjects carrying CT/TT alleles and 49 subjects carrying CC allele. The gender, education, and APOE ε4 status were matched between genotypes. According to the threshold of expected cluster size (90) obtained from the SPM results, we found that several regions exhibited a notably different rate of change of the GMV with aging between the two groups (Table 2, Figure 1). After FDR correction for multiple comparisons, only the right middle temporal pole (MTP) (Figure 2A) retained a significant group × age interaction effect on the GMV (Cluster Size = 63, FDR-corrected, P< 0.05), which demonstrated a difference in the regression slope between volume and age (Figure 2B). The GMV of this cluster within the CT/TT group decreased with age, while the CC group did not show similar effect. No regions could survive the multiple comparison correction during the analysis of the main group effect.
Table 2.
Areas of GMV Significantly Affected by the Interaction between Genotypes and Age
| Location* | Cluster Size | t-value | Z value | MNI coordinates (mm)† |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Middle Temporal Pole (R) | 698 | 5.22 | 4.80 | 38 | 12 | −47 |
| Hippocampus (L) | 265 | 5.12 | 4.72 | −24 | −15 | −6 |
| Middle Temporal Pole (L) | 389 | 4.50 | 4.22 | −45 | 15 | −41 |
| Thalamus (R) | 675 | 4.23 | 4.00 | 12 | −1 | −9 |
| Inferior Frontal Gyrus_pars opercularis (R) | 194 | 4.21 | 3.98 | 57 | 15 | 25 |
| Middle Temporal Gyrus (R) | 143 | 4.16 | 3.93 | 60 | −57 | 18 |
| Inferior Parietal Gyrus (L) | 145 | 4.02 | 3.81 | −54 | −33 | 43 |
| Inferior Frontal Gyrus_pars orbitalis (R) | 211 | 3.93 | 3.74 | 50 | 23 | −6 |
| Superior Temporal Pole (R) | 123 | 3.85 | 3.66 | 63 | 3 | −2 |
| Supplementary Motor Area (L) | 136 | 3.82 | 3.64 | −5 | −12 | 63 |
| Superior Frontal Gyrus (R) | 195 | 3.72 | 3.55 | 23 | −7 | 66 |
Abbreviations: R, right; L, left.
Areas with a cluster size exceeding 90 (the expected cluster size according to the SPM results) are displayed.
The “X Y Z” denotes the coordinates of the primary peak locations in the MNI space.
Figure 1.
Areas of GMV significantly affected by the interaction between genotypes and age without multiple comparison correction are presented, including the right middle temporal pole, left hippocampus, left middle temporal pole, right thalamus, right inferior frontal gyrus (pars opercularis), right middle temporal gyrus, left inferior parietal gyrus, right inferior frontal gyrus (pars orbitalis), right superior temporal pole, left supplementary motor area and right superior frontal gyrus. The area in the blue circle is the right middle temporal pole, which could withstand false discovery rate correction (P< 0.05).
Figure 2.
Imaging graphic of the right middle temporal pole and slope changes of its gray matter volume with aging stratified by genotypes. (A) After false discovery rate correction (P< 0.05), the right middle temporal pole retained a significant interaction effect with a cluster size of 63. (B) The significance of the interaction effect was reflected in the difference between the regression slopes of the two groups. Furthermore, the slope of the CT/TT group significantly differed from 0 (F = 31.33, P< 0.001), while the slope of the CC group did not (F = 0.1, P = 0.758).
3.3 The relationships between the GMV and cognitive tests
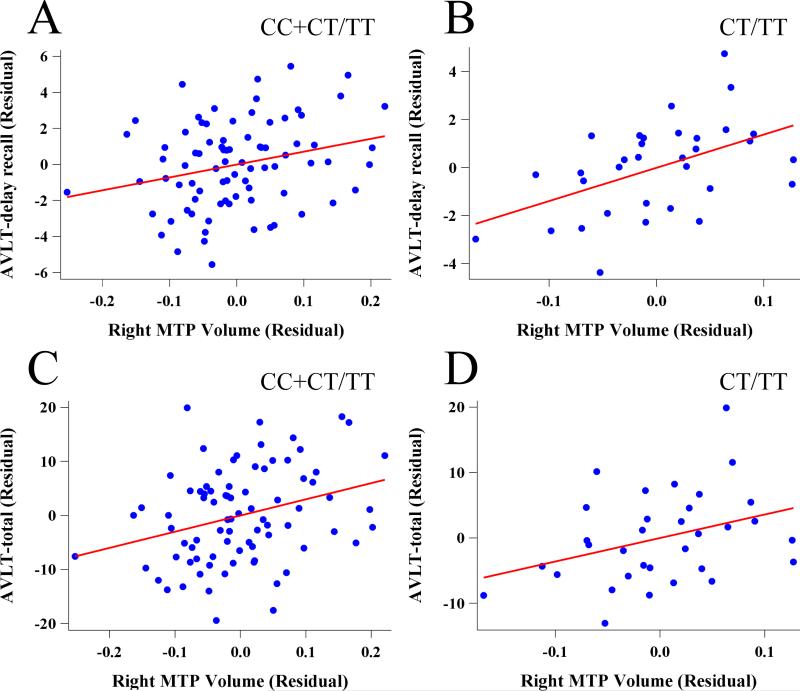
The GMV extracted from significant interaction areas were employed in the following partial correlation analyses. The results showed that the GMV in the right MTP significantly positively related to the AVLT-delay recall (r = 0.25, P = 0.028) and the AVLT-total (r = 0.30, P = 0.008) scores. Furthermore, significant correlations with AVLT-delay recall (r = 0.56, P = 0.002) and AVLT-total (r = 0.42, P = 0.023) were also observed in the CT/TT group. Nevertheless, the two memory indicators of the CC group did not significantly correlate with GMV in the right MTP (AVLT-delay recall: r = 0.04, P = 0.771, AVLT-total: r = 0.14, P = 0.346). To adjust for the covariates, the residual was calculated to display the correlation between the neuropsychological tests and the GMV (Figure 3) [30]. Spearman partial correlation analyses were applied because of the non-normal distribution of AVLT-delay recall (P = 0.0077) and AVLT-total (P = 0.0459) scores.
Figure 3.
Correlations between the neuropsychological tests and gray matter volume in the right middle temporal pole. (A) The right middle temporal pole volume positively correlated with the AVLT-delay recall in all subjects (r = 0.25, P = 0.028). (B) The right middle temporal pole volume positively correlated with the AVLT-delay recall in the CT/TT group (r = 0.56, P = 0.002). (C) The right middle temporal pole volume positively correlated with the AVLT-total in all subjects (r = 0.30, P = 0.008). (D) The right middle temporal pole volume tended to positively correlate with the AVLT-total in the CT/TT group (r = 0.42, P = 0.023). AVLT, Auditory Verbal Learning Test.
4. Discussion
In this study, we first examined the effects of the SORL1 gene rs1699102 polymorphism on age-related cognitive decline and cortical GMV reduction in a non-demented elderly Chinese Han population. Some studies have investigated the relationship between the variants in the SORL1 gene and cognitive function. However, the findings were somewhat mixed [10, 11, 13, 31]. Several demographic factors may modulate the effects of SORL1 on cognitive abilities, which could cause these discrepant findings. Aging is a well-known risk factor for developing AD and cognitive impairment. Previous studies have suggested that age may interact with other genetic factors to affect cognition during aging [32]. Reynolds and colleagues first found that several SORL1 SNPs are associated with cognitive change trajectories in older adults across multiple domains, including the spatial domain, episodic memory and verbal abilities [12]. Unfortunately, the SNP rs1699102 was excluded from the analysis in their study because of genotyping failure. In the current study, the polymorphism was found to be associated with age-related cognitive decline, primarily in episodic memory and processing speed. The performance of the AVLT, ROCF Recall and TMT-A tests in the T allele carriers declined at a faster rate compared with the CC group. An impairment in episodic memory is the primary symptom of AD and amnestic mild cognitive impairment and can be used to predict the likelihood of progression to dementia [33]. The speed of cognitive processing is considered a fundamental part of the cognitive system. A decrease in the processing speed impairs the fluid cognitive abilities [34]. Our findings suggest that the T allele of the rs1699102 polymorphism may be associated with a steeper age-related decline in cognitive function and a higher risk of AD. This hypothesis is consistent with a study in Chinese subjects, which reported that the T allele is more abundant in AD than in controls, although this difference is not significant [9]. The relationship of rs1699102 polymorphism with cognitive aging and AD requires further confirmation in other ethnic groups as well as a larger sample of the Chinese Han population.
Global or regional GM atrophy is a common change during aging that seems to be related to the impairment of multiple cognitive domains. In the current study, the rs1699102 polymorphism was found to be associated with the age-related reduction of the right MTP. A faster volume reduction of this region was observed in carriers of the T allele than in CC genotype carriers, with similar trends in several frontal, temporal and limbic regions (but not significant after correction for multiple comparisons). The temporal pole was atrophied in patients with AD [35]. The right temporal pole is believed to store personal memory, and showed activated during the discrimination of familiar faces and scenes from unfamiliar ones, and this region is likely involved in the recognition of familiar objects [36]. Moreover, the right anterior temporal pole activation was observed to reflect the psychological set associated with emotional memory retrieval [37]. In our study, the GMV in the right MTP and the episodic memory scores were found to significantly associate in all the subjects, and this relationship was still significant in the CT/TT carriers. Our findings suggest that non-demented elderly Han Chinese carriers of the rs1699102 T allele are at a higher risk of GM atrophy in the right MTP than individuals homozygous for the C allele, and this risk may be associated with the poorer performance of episodic memory tests. This association may explain the impairment of episodic memory in the T-allele carriers.
To our knowledge, only one study has investigated the effect of the SORL1 gene rs1699102 polymorphism on brain volume and did not find a significant relationship between this SNP and any of the MRI measures, including general cerebral atrophy, hippocampal atrophy, white matter hyperintensities and overall cerebrovascular disease [16]. However, the haplotypes that encompass the SNP rs1699102 were associated with hippocampal atrophy and general cerebral atrophy. Similar to their findings, the regional volume did not differ between genotype groups in our study, which may suggest that this SNP only slightly affects the GMV. The interaction between age and the polymorphism affected the left hippocampus volume, but this effect was not significant after FDR correction. We note that the current study enrolled non-demented elderly subjects, unlike the AD patients and unaffected siblings using in the aforementioned study. In addition, our results were based on the whole-brain analyses, while only the local regions of the GM were examined in that study. Further studies of larger samples and various ethnic populations are required to delineate the relationship of the rs1699102 polymorphism with brain atrophy during aging.
We are aware of several methodological limitations in this study. First, only the SNP rs1699102 but not other SORL1 SNPs or haplotypes were singled out. The relatively very mild effect of the polymorphism on regional brain volume may in part be due to this selection. Second, the genetic effects on age-related cognitive decline and cortical GMV reduction identified in this study must be interpreted with caution due to the cross-sectional design of this study, which may result in a confounding effect because of the cohort effect. A prospective longitudinal study is needed to confirm these results. Third, the SorLA expression in the brain was not measured in this study, and this measurement may be necessary to clarify the exact mechanisms of the association between SORL1 variants and age-related brain atrophy.
5. Conclusions
In conclusion, we observed the effects of the SORL1 gene rs1699102 polymorphism on the age-related cognitive declines in episodic memory and processing speed, as well as a GMV reduction of the right MTP in a non-demented elderly Chinese Han population. Carriers of the rs1699102 T allele showed an accelerated age-related cognitive decline and GMV reduction compared with the CC group. These findings not only provide insight into how the SORL1 variants shape the neural system to modulate cognitive decline but also support the hypothesis that SORL1 may represent a candidate gene for late-onset AD.
Acknowledgements
This work was supported by the State Key Program of National Natural Science of China (grant number: 81430100), the Beijing New Medical Discipline Based Group (grant number 100270569), the Natural Science Foundation of China (grant number 81173460, 81274001 and 31500925), and the National Institute on Aging (grant number R01AG031581 and P30AG19610).
Footnotes
Author's contributions
He Li, Chenlong Lv and Caishui Yang analyzed the data and drafted the manuscript. Dongfeng Wei assisted with the data collection and analysis. Kewei Chen advised on biostatistical methodology and critically reviewed the manuscript. Zhanjun Zhang conceived the original idea for the study. Zhanjun Zhang and Shaowu Li supervised in the conception, and revised the manuscript. All authors read and approved the final manuscript.
Disclosure statement
The authors of this manuscript have no conflicts of interest to declare.
References
- 1.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 2.Jiang T, Yu JT, Tian Y, Tan L. Epidemiology and etiology of Alzheimer's disease: from genetic to non-genetic factors. Curr Alzheimer Res. 2013;10:852–867. doi: 10.2174/15672050113109990155. [DOI] [PubMed] [Google Scholar]
- 3.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 4.Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Offe K, Dodson SE, Shoemaker JT, et al. The lipoprotein receptor LR11 regulates amyloid beta production and amyloid precursor protein traffic in endosomal compartments. J Neurosci. 2006;26:1596–1603. doi: 10.1523/JNEUROSCI.4946-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng Y, Lee JH, Cheng R, St George-Hyslop P, Mayeux R, Farrer LA. Association between SORL1 and Alzheimer's disease in a genome-wide study. Neuroreport. 2007;18:1761–1764. doi: 10.1097/WNR.0b013e3282f13e7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caglayan S, Bauerfeind A, Schmidt V, et al. Identification of Alzheimer disease risk genotype that predicts efficiency of SORL1 expression in the brain. Arch Neurol. 2012;69:373–379. doi: 10.1001/archneurol.2011.788. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Chulikavit M, Pang D, Zigman WB, Silverman W, Schupf N. Association between genetic variants in sortilin-related receptor 1 (SORL1) and Alzheimer's disease in adults with Down syndrome. Neurosci Lett. 2007;425:105–109. doi: 10.1016/j.neulet.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan EK, Lee J, Chen CP, Teo YY, Zhao Y, Lee WL. SORL1 haplotypes modulate risk of Alzheimer's disease in Chinese. Neurobiol Aging. 2009;30:1048–1051. doi: 10.1016/j.neurobiolaging.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Seshadri S, DeStefano AL, Au R, et al. Genetic correlates of brain aging on MRI and cognitive test measures: a genome-wide association and linkage analysis in the Framingham Study. BMC Med Genet. 2007;8(Suppl 1):S15. doi: 10.1186/1471-2350-8-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houlihan LM, Harris SE, Luciano M, et al. Replication study of candidate genes for cognitive abilities: the Lothian Birth Cohort 1936. Genes Brain Behav. 2009;8:238–247. doi: 10.1111/j.1601-183X.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds CA, Zavala C, Gatz M, et al. Sortilin receptor 1 predicts longitudinal cognitive change. Neurobiol Aging. 2013;34:1710, e1711–1718. doi: 10.1016/j.neurobiolaging.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu F, Ikram MA, Janssens AC, et al. A study of the SORL1 gene in Alzheimer's disease and cognitive function. J Alzheimers Dis. 2009;18:51–64. doi: 10.3233/JAD-2009-1137. [DOI] [PubMed] [Google Scholar]
- 14.Bralten J, Arias-Vasquez A, Makkinje R, et al. Association of the Alzheimer's gene SORL1 with hippocampal volume in young, healthy adults. Am J Psychiatry. 2011;168:1083–1089. doi: 10.1176/appi.ajp.2011.10101509. [DOI] [PubMed] [Google Scholar]
- 15.Felsky D, Szeszko P, Yu L, et al. The SORL1 gene and convergent neural risk for Alzheimer's disease across the human lifespan. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.K TC, Lunetta KL, Baldwin CT, et al. Association of distinct variants in SORL1 with cerebrovascular and neurodegenerative changes related to Alzheimer disease. Arch Neurol. 2008;65:1640–1648. doi: 10.1001/archneur.65.12.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Ma C, Zhang J, et al. Prevalence of and potential risk factors for mild cognitive impairment in community-dwelling residents of Beijing. J Am Geriatr Soc. 2013;61:2111–2119. doi: 10.1111/jgs.12552. [DOI] [PubMed] [Google Scholar]
- 18.Zhang MY, Katzman R, Salmon D, et al. The prevalence of dementia and Alzheimer's disease in Shanghai, China: impact of age, gender, and education. Ann Neurol. 1990;27:428–437. doi: 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- 19.Guo Q, Zhao Q, Chen M, Ding D, Hong Z. A comparison study of mild cognitive impairment with 3 memory tests among Chinese individuals. Alzheimer Dis Assoc Disord. 2009;23:253–259. doi: 10.1097/WAD.0b013e3181999e92. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YLJ, Guo QH, Hong Z. Rey-Osterriche complex figure test used to identify mild Alzheimer's disease. Chin J Clin Neurosci. 2006;14:501–504. [Google Scholar]
- 21.Sheridan LK, Fitzgerald HE, Adams KM, et al. Normative Symbol Digit Modalities Test performance in a community-based sample. Arch Clin Neuropsychol. 2006;21:23–28. doi: 10.1016/j.acn.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Lu JCCG, Hong Z, Shi WX, Lu CZ. Trail making test used by Chinese elderly patients with mild cognitive impairment and mild Alzheimer dementia. Chinese Journal of Clinical Psychology. 2006;4:118–121. [Google Scholar]
- 23.Ishiai S, Sugishita M, Ichikawa T, Gono S, Watabiki S. Clock-drawing test and unilateral spatial neglect. Neurology. 1993;43:106–110. doi: 10.1212/wnl.43.1_part_1.106. [DOI] [PubMed] [Google Scholar]
- 24.Guo QHHZ, Shi WX, Sun YM, Lv CZ. Boston naming test using by Chinese elderly, patient with mild cognitive impairment and Alzheimer's dementia. Chinese Mental Health Journal. 2006;20:81–85. [Google Scholar]
- 25.Mok EH, Lam LC, Chiu HF. Category verbal fluency test performance in chinese elderly with Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;18:120–124. doi: 10.1159/000079190. [DOI] [PubMed] [Google Scholar]
- 26.QH GZH, CZ L, Y Z, JC L, D D. Application of Stroop color-word test on Chinese elderly patients with mild cognitive impairment and Alzheimer's dementia. Chin J Neuromed. 2005;4:701–704. [Google Scholar]
- 27.Takei N, Miyashita A, Tsukie T, et al. Genetic association study on in and around the APOE in late-onset Alzheimer disease in Japanese. Genomics. 2009;93:441–448. doi: 10.1016/j.ygeno.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 29.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Limongi R, Tomio A, Ibanez A. Dynamical predictions of insular hubs for social cognition and their application to stroke. Front Behav Neurosci. 2014;8:380. doi: 10.3389/fnbeh.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shulman JM, Chibnik LB, Aubin C, Schneider JA, Bennett DA, De Jager PL. Intermediate phenotypes identify divergent pathways to Alzheimer's disease. PLoS One. 2010;5:e11244. doi: 10.1371/journal.pone.0011244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattay VS, Goldberg TE, Sambataro F, Weinberger DR. Neurobiology of cognitive aging: insights from imaging genetics. Biol Psychol. 2008;79:9–22. doi: 10.1016/j.biopsycho.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blacker D, Lee H, Muzikansky A, et al. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007;64:862–871. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- 34.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 35.Arnold SE, Hyman BT, Van Hoesen GW. Neuropathologic changes of the temporal pole in Alzheimer's disease and Pick's disease. Arch Neurol. 1994;51:145–150. doi: 10.1001/archneur.1994.00540140051014. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura K, Kawashima R, Sato N, et al. Functional delineation of the human occipito-temporal areas related to face and scene processing. A PET study. Brain. 2000;123(Pt 9):1903–1912. doi: 10.1093/brain/123.9.1903. [DOI] [PubMed] [Google Scholar]
- 37.Dolan RJ, Lane R, Chua P, Fletcher P. Dissociable temporal lobe activations during emotional episodic memory retrieval. Neuroimage. 2000;11:203–209. doi: 10.1006/nimg.2000.0538. [DOI] [PubMed] [Google Scholar]