Abstract
Background
Cancer-related fatigue (CRF) is one of the most common symptoms among women with recurrent ovarian cancer, yet it remains extremely difficult to manage. Symptom management typically requires patients to set goals and strategies to manage their CRF, but little is known about how to create individualized CRF symptom management goals and strategies.
Objective
To describe cancer patients’ goals and strategies for managing cancer-related fatigue along with their process of individualizing both.
Methods
This study is a qualitative analysis with supportive quantitative description of a web-based symptom management RCT, the WRITE Symptom Study. Researchers conducted a content analysis on N=47 participants’ CRF symptom care-plans to identify common themes in participants’ goals, categorize strategies, and describe the individualization process.
Results
Four general themes were identified among participants’ CRF goals: 1) Enjoying time with friends and family, 2) Doing the things I enjoy, 3) Having energy to be physically active, and 4) Keeping up with what I need to do. CRF strategies were categorized into 13 groups including conserving energy, increasing activity, and talking with healthcare providers. A multistep individualization process resulted in personally-meaningful strategies.
Conclusions
The process by which participants individualized their CRF strategies consisted of identifying, confirming, testing, and evaluating different CRF strategies and resulted in refined, specific and individualized strategies intended to eventually ensure participants achieve their goal.
Implications for Practice
Clinicians can assist patients in individualizing their CRF goals and strategies. Individualization of CRF goals and strategies assists patients in visualizing how improving CRF will impact their life.
Background
Goal-setting is a key means by which patients participate and engage in health behavior change.1–4 Frequently-cited outcomes of goal-setting include improved symptom management, weight reduction, and diabetes management.5,6 Despite acknowledging the importance of setting individualized goals, inadequate attention has been given to the critical process of identifying and enacting individualized patient goals.7
Symptom management is an area of clinical care and oncology nursing typically driven by patient goals for improvement. Symptoms frequently cause distress and interference in patients’ daily lives, motivating patients to seek relief through consultation with their health care team and implementation of self-management strategies. Gaining an understanding of patients’ processes of individualizing their symptom management efforts to meet their unique needs can benefit clinicians and researchers aiming to help patients improve symptom management.
Cancer-related fatigue (CRF) is not only one of the most severe and distressing symptoms among cancer survivors but also one of the most difficult to manage.8,9 CRF is defined as tiredness or exhaustion not resulting from activity (or out of proportion to the level of activity) and not relieved by rest.10 It can be caused by the cancer itself as well as by comorbid conditions, treatment side effects, sleep disturbances, and/or psychological distress. Due to its universal prevalence and need for treatment, CRF was accepted into the International Classification of Diseases in 1999.11
Women with ovarian cancer are known to experience high levels of fatigue both during and after treatment.12 Ovarian cancer is the fifth deadliest cancer for women in the U.S. 12 Most women are diagnosed with ovarian cancer at Stage III when treatment is less effective and the 5-year survival rate is only 39%.13 Overall, 70–90% of all patients with ovarian cancer will experience a recurrence,14 and the median survival time after a recurrence is only 12–24 months.15 For these reasons, effectively learning how to manage CRF is particularly needed within the recurrent ovarian cancer patient population to improve women’s quality of life.
With an overall prevalence of 48% among all cancer survivors,8 CRF is high during treatment and often persists afterward.16 While non-pharmacological interventions such as exercise, restorative activities, and establishing a balance between sleep and activity have been shown to reduce CRF, these all require significant time and effort on the part of the patient. CRF management therefore requires ongoing individualization to minimize burden while increasing the likelihood of achieving the patient’s desired goals particularly in light of the limitations imposed by the underlying disease and symptom burden.
Purpose
The purpose of this study is to describe how women with recurrent ovarian cancer participating in a nurse-delivered symptom management intervention individualize standard CRF symptom management strategies to meet their personal goals. The aims of this study are to (1) describe participants’ CRF goals, (2) categorize participants’ chosen strategies for achieving their goals, and (3) describe the process of individualizing goals and strategies in accordance with participants’ unique motivations and priorities.
Methods
Design and Sample
The current study is a qualitative analysis with support from quantitative data of the WRITE Symptoms Study. The parent study, the Written Representational Intervention to Ease (WRITE) Symptoms trial (Gynecological Oncology Group (GOG) - 0259), is national randomized clinical trial (RCT) to improve symptom distress among women with recurrent ovarian cancer. This three-arm study tests the efficacy of a web-based, asynchronous nurse-delivered vs. patient self-directed psycho-educational intervention compared to usual care to improve symptom distress and symptom communication. The intervention is based on the Representational Approach to patient education in which patients’ representations of their illness, symptoms, and symptom management were assessed in order to identify concerns and barriers to symptom management and to explore ways in which participants could gain better control over their symptoms.17 The WRITE Symptoms intervention includes the development, subsequent refinement, and ultimate implementation of goals and strategies for addressing the top three symptoms over which participants wanted to gain better control. Detailed information regarding the parent study is reported elsewhere.18 The Institutional Review Board at the University of Pittsburgh approved the study.
Once the clinical research coordinators at GOG conducted the informed consent process with the patient, the patient was entered into the WRITE Symptoms Internet baseline assessment system and was assisted by the coordinators in creating a username and password. The patient was then able to log in and complete baseline questionnaires. Only after submitting completed questionnaires were patients officially registered to the study, enrolled in the full WRITE Symptoms system, and randomized to treatment condition. Inclusion criteria for the parent study included being diagnosed with recurrent or persistent ovarian, fallopian, or primary peritoneal cancer and experiencing 3 cancer- or treatment-related symptoms. For the current analysis, inclusion criteria included: (a) randomized to the nurse-delivered intervention arm; (b) selected fatigue as one of their three target symptoms; and (c) completed a Symptom Care Plan for fatigue. Symptom Care Plans of eligible participants were extracted for qualitative analysis to address the study aims.
Intervention
Women randomized to the nurse-delivered WRITE Symptoms study arm were paired with a nurse interventionist who was not a member of participants’ health care team. The participant and nurse interventionist asynchronously communicated via message boards to develop, individualize, and implement a Symptom Care Plan to address their CRF. Each participant was assigned to one of four nurse interventionists for the entirety of the eight-week intervention. Participants were provided an evidence-based, nurse interventionist-developed symptom care guide that included causes, prevention methods, management strategies, and outside resources specific to CRF.
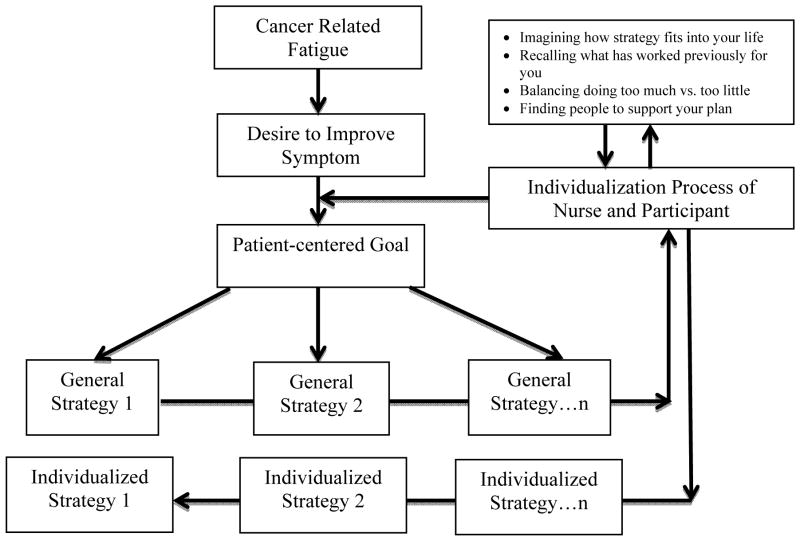
Two key features of participants’ Symptom Care Plans were the selection of a patient-centered goal and the corresponding strategies designed to achieve that goal. These were developed through an iterative process in which the nurse and participant worked together to choose and refine strategies the participant deemed both feasible and relevant to helping them attain their goal. The SMART (Specific, Measurable, Achievable, Relevant, Timely) acronym was used to assist participants in developing their overarching goals regarding managing their CRF. The strategies were intended to be short-term, explicit, attainable tasks that, when achieved, would support attainment of the goal. Participants’ strategies might contain elements of the strategies listed in the provided care guide, but the nurse interventionist encouraged participants to refine these standard strategies to create highly individualized strategies. Once developed and refined, participants’ goals and strategies were documented in their individual Symptom Care Plan. The figure illustrates the process of goal and strategy development as it relates to CRF.
Figure.
Process of Individualizing Cancer Related Fatigue Symptom Management
Measures
Baseline measures from the WRITE Symptoms Study are included in this analysis. Socio-demographic variables were assessed using the Center for Research in Chronic Disorders Survey.19 CRF and additional symptoms were measured using the Symptom Representation Questionnaire. This questionnaire includes 28 commonly reported cancer- and treatment-related symptoms specific to ovarian cancer. Participants ranked each symptom’s severity when it was at its worst in the past week on an 11-point ranking scale ranging from 0 (“did not have the symptom”) to 10 (“as bad as I can imagine”). This scale has demonstrated strong reliability and validity within the ovarian cancer population including strong subscale internal consistency (α= 0.63 – 0.88).20
Data Analysis
Goals and Strategies
Two nurse researchers including a nurse interventionist (initials withheld for blind review) and a graduate student researcher (initials withheld for blind review) conducted a descriptive content analysis of participants’ Symptom Care Plans.21(pp26–53),22 Our goal was to describe the content of participants’ explanations of their goals and strategies included in their Plans and not to derive overarching themes. We purposefully did not enter the analysis with a preconceived structure so that common descriptions could organically emerge. We analyzed participants’ goals and strategies using the same analytic process. First, we independently read and described participants’ Plans and then met in person to compare findings. If we disagreed on how to describe goals or strategies, then we discussed our respective rationales and came to consensus. This process resulted in categories of participants’ self-selected goals and strategies to manage their CRF.
Individualization Process
We then reviewed Symptom Care Plans to uncover the processes of individualization. We independently read a subset of Symptom Care Plans, coded the text for identifications of processes used by participants, and summarized the codes with initial overarching descriptions and possible themes of these processes. We then met together to share our independent codes and themes, discuss any disagreements, and consider alternate conclusions to our analyses. We wrote memos at each stage to provide an audit trail for how decisions about themes and groupings were made. After multiple rounds of analysis for additional subsets of participants’ Symptom Care Plans, we achieved consensus on the themes of the individualization process. An additional graduate student researcher (initials withheld for blind review) audited the analysis to ensure the findings were supported by the data, and the principal investigator of the study (initials withheld for blind review) confirmed findings at the end of the analysis. This analysis resulted in themes of the process participants’ underwent to individualize CRF management strategies.
Participants’ strategies frequently included several distinct aspects that clearly overlapped between more than one strategy category. If this occurred, then we counted a participant’s strategy under both strategy categories in order to account for the breadth of her response. For example, one participant listed the strategy of “Use my deep breathing exercises as needed for getting back to sleep and reducing anxiety,” in the analysis, and we counted this as a strategy addressing both rest/sleep and emotional distress.
Exemplars of participant goals and strategies were selected to represent the tone and themes reported by all participants. Given the lack of further observations or text on which to base a thick description of the goals and strategies, exemplars also were chosen based on their richness of content and completeness of thought. Due to their richness, exemplars often connected many ideas, actions, and wishes the participant had to manage her CRF. These exemplars may have been counted within different categories, but are presented to provide context and to relay the depth and intensity by which participants developed their individualized strategies.
We quantitatively analyzed CRF strategies by counting the number of unique strategies per participant, per category of strategy, and how many times participant strategies within a one category overlapped with a separate category (e.g. if that strategy was counted twice). We also calculated means and standard deviations of participants’ CRF and total symptom severity for descriptive purposes. Finally, we reviewed participants’ socio-demographic characteristics using descriptive statistics. We used SPSS version 23 for all descriptive statistics.
Results
Forty-seven (N=47) participants met eligibility criteria for this analysis. Table 1 reports sample demographic and health characteristics. The average age of the sample was 57.35 (SD = 8.22; Observed range = 34 – 73) and participants had an average of 15.74 (SD = 2.81) years of formal education. Forty-six percent of women were married or living with a partner, 93.6% self-identified as White, 31.1% had an annual household income less than $30,000, and only 31.9% were employed full-time. Women were an average of 45.10 (SD = 37.79) months since their cancer diagnosis, and 79.1% of women were receiving treatment at the time of the intervention. This sub-group of women completing care plans for CRF reflected the demographics of the full parent study sample, except that they had a significantly higher number of years of formal education [t(478)=2.70, p = 0.007].
Table 1.
Sample Characteristics (N = 47)
| M ± SD | Range | N | % | |
|---|---|---|---|---|
| Demographic | ||||
| Age | 57.35 ± 8.22 | 34 – 73 | ||
| Years of education | 15.74 ± 2.81 | 11 – 21 | ||
| Race | ||||
| White | 44 | 93.6 | ||
| African American | 1 | 2.1 | ||
| American Indian | 1 | 2.1 | ||
| Other | 1 | 2.1 | ||
| Employment status | ||||
| Working full-time | 15 | 31.9 | ||
| Retired, not working at all | 12 | 25.5 | ||
| Disabled/unable to work | 8 | 17.0 | ||
| Working part-time | 4 | 8.5 | ||
| Other | 8 | 17.0 | ||
| Annual household income | ||||
| < $30,000 | 14 | 31.1 | ||
| $30–69,999 | 23 | 46.7 | ||
| $70–99,999 | 4 | 8.8 | ||
| ≥ $100,000 | 6 | 13.3 | ||
| Marital status | ||||
| Currently married/living with significant other | 21 | 45.7 | ||
| Never married | 12 | 26.1 | ||
| Separated/divorced | 7 | 15.2 | ||
| Other | 6 | 12.8 | ||
| Health | ||||
| Currently receiving treatment | 37 | 79.13 | ||
| Months since cancer diagnosisa | 45.10 ± 37.79 | |||
| Mean fatigue score | 6.34 ± 2.40 | 1 – 10 | ||
| Sum symptom severity score | 60.85 ± 29.27 | 17 – 128 | ||
| Mean symptom severity score | 2.17 ± 1.05 | 0.61 – 4.57 | ||
The range of months since diagnosis is not available.
At baseline, women in this sample had a mean fatigue severity score of 6.34 ± 2.40 out of 10 (Observed Range = 1–10) indicating moderate fatigue levels. Participants reported a mean Symptom Representation Questionnaire sum symptom severity score of 60.85 ± 29.27 out of 280 across the 28 symptoms, and a mean symptom severity score of 2.17 ± 1.05 out of 10. By comparison, the rest of the parent study sample (N = 452) had similar symptom severity scores with sum symptom severity scores of 57.95 ± 32.57, and a mean symptom severity score of 2.07 ± 1.16. Mean fatigue scores were lower in the parent study sample compared to this subsample (M=5.09 ± 2.78) [t(498)=2.81, p = 0.003], a finding consistent with these women having chosen CRF as a target symptom.
Individualized Goals
Fatigue goals are listed and described in Table 2 and reflected four major themes: (1) Enjoying time with friends and family, (2) Doing the things I enjoy, (3) Having energy to be physically active, and (4) Keeping up with what I need to do. Women focused their symptom management goals on increasing their level of activity and productivity so that they could enjoy friends and family, accomplish valued tasks, and avoid the inactivity associated with CRF.
Table 2.
Cancer-Related Fatigue Goals
| Goal Themes | Example |
|---|---|
| Enjoying time with friends and family |
“For the 2 weeks after this next chemo, I would like to restore my energy so my fatigue does not prevent me from spending more quality time relaxing with my family and maybe going out for some evening entertainment.” “To be actively ‘involved’ in life; … to be able to have my grandchildren visit with my husband and I every weekend as they normally do.” |
| Doing the things I enjoy |
“My goal during the next month is to reduce my level of fatigue … so that I will feel more energized and able to do the things I want to do.” “Have more energy to do the things that are important to me, such as: work with my church, helping my family, riding horseback, and napping less.” |
| Having energy to be physically active |
“I want to do a 3-mile walk for the National Ovarian Cancer Coalition next Spring.” “During the next 2 weeks my goal is to reduce the level of my fatigue so that I can continue some kind of exercise daily without taking backward steps on the days that I really don’t feel like moving.” |
| Keeping up with what I need to do |
“To do all my every day chores without feeling exhausted.” “To feel good about being able to accomplish a few tasks around the house (like making my bed, fixing a meal and/or taking a walk outside) without the exhaustion taking over.” |
Individualized Strategies
Table 3 lists the strategies along with the number of times each strategy was selected, whether the strategy overlapped with other strategies, and exemplar quotations for each strategy. Participants selected 358 unique strategies to manage CRF with a mean of 7.6 unique strategies per participant (Observed range = 2–44). Strategies were considerably more varied than participant goals and could be grouped into 13 general categories. Participants had a mean of 1.2 (Observed range = 0–5) strategies that overlapped between categories, indicating that most strategies fit into a single category.
Table 3.
Cancer-Related Fatigue Strategiesa
| Strategy Category | # Selected | # Overlapping | Keywords | Quotation Providing Example of Individualization |
|---|---|---|---|---|
| 1. Energy Conservation | 98 | 24 | Prioritizing; Planning; Pacing | “New grocery shopping plan: A) Divide shopping trips into short but more frequent trips. B) Use these more frequent trips as way to increase activity in a structured way to build endurance.” |
| 2. Activity | 53 | 7 | Walking; Exercising | “I will begin to walk at least 20 minutes per day 3–4 days a week. This should be easy for me to do as I live in a very ‘walkable’ area and my husband is agreeable to walking with me.” |
| 3. Talk with Health Care Team | 47 | 11 | Discussing and sharing plan of care; Getting a referral | “Talk with my doctor…about my fatigue and a referral to a nutritionist who can help develop a safe, healthy, balanced nutrition plan that can be effective for my symptoms of fatigue and weight gain.” |
| 4. Rest/Sleep | 37 | 16 | Accepting that sleep is disturbed; Planning for rest; Not pushing self | “When I do feel very tired in the AM, I will allow myself to rest for 30–60 minutes. (I really dislike doing this because the rest usually gets to be more than an hour! But will try it again.) I know I feel better after I rest rather than pushing through the tiredness.” |
| 5. Nutrition | 39 | 12 | Planning meals; Drinking fluids; Eating healthy food | “I am usually very conscientious about drinking water…but on those days after chemo it is very hard…. New Strategy: drink 4 cups of fluid (if water won’t go down, Fresca usually works) by 1 PM, then 4 more cups by 10 PM.” |
| 6. Dealing with Emotions | 28 | 9 | Relaxing; Self-care | “Attend retreat with church group on 9/27 to help calm and cleanse mind.” |
| 7. Talk with Friends & Family | 29 | 16 | Asking for support; Accepting support |
“Limit social visits and concentrate on being with people I like to be with. Otherwise not be afraid to tell visitors that I am tired and need to rest. “ “With my boss’ permission and support I am going to work from home occasionally and also take an afternoon off a couple times a month.” |
| 8. Energy Restoration | 20 | 4 | Listening to music; Being in nature; Reading; Breathing | “Spend time reading, listening to music, sitting and contemplating - all of those activities have been energizing in the past and I will endeavor to incorporate them back into my life.” |
| 9. Medicine | 22 | 9 | Taking medications | “Take pain and nausea medicine on schedule daily to reduce symptoms and their effect on fatigue. |
| 10. Prevention & Monitoring | 15 | 3 | Keeping diary; Track; Find patterns | “I’m going to try to carry something in my purse to keep a symptom journal and take it out when I am in the Dr’s office.” |
| 11. Other | 13 | 2 | Using available supports |
“Continue stopping by chapel in hospital, after paracentesis to rest and pray.” “Talk with [a] Patient Assistant about financial help with medications.” |
| 12. Pain Control | 7 | 4 | Taking and changing medications; Non- pharmacological | “Consider adjusting thoughts on using pain medicines to help reduce pain, and therefore fatigue. |
| 13. Coping/Acceptance | 2 | 1 | Acknowledging limitations; Accepting current state | “I am going to try to be content with what I do accomplish even though the end may be another day. I can win the game tomorrow just as well as I can win today.” |
The symptom care guide for CRF described several strategies for managing fatigue including physical activity, energy conservation (planning, prioritizing, pacing, and positioning), energy restoration, rest, pain control, emotional distress management, nutrition, talking with friends and family, talking with the health care team, and pharmacologic approaches.
Of the 358 unique strategies to improve CRF, the most frequently selected strategies reflected the participants’ desires to conserve energy (n = 98, 27.3%), increase levels of activity (n = 53, 14.8%), and talk with their health care team (n = 47, 13.1%). Conserving energy by prioritizing, planning, and pacing their day in order to accomplish tasks of daily living was listed in the written symptom care guide provided to all patients, and women frequently discussed altering their daily cooking, cleaning, and other household activities to accomplish specific, manageable pieces without exhausting themselves.
The second most common strategy was increasing activity levels, mostly through walking and exercising at moderate levels. The strategy of talking with the health care team was also chosen frequently; this strategy was viewed both as a means of informing the team of the severity of the CRF and as a means of asking for symptom management recommendations or seeking referral for to a health care specialist (e.g. physical therapy, health psychology, etc.) to obtain additional support or intervention.
With the help of their nurse interventionist, women frequently individualized strategies by selecting multiple pre-written strategies from the symptom care guides provided by the study and combining them into a single strategy. Some women created unique strategies not listed in symptom care guide which we listed as “other” since they were infrequently selected. Spiritual coping including praying and/or attending religious services was one of the most common unique strategies reported by women.
Process of Individualizing Goals and Strategies
Participants’ process of individualizing standard CRF recommendations alongside their nurse interventionist followed the path illustrated in the figure. Beyond simply experiencing CRF, women needed the desire and ability to improve that symptom. Generally, individualization began with a participant engaging in an exploration of the cognitive and emotional factors that influence symptom management, as is prescribed in the Representational Approach that guided the intervention.22 The nurse’s steps in this exploration included asking a participant to (1) imagine how the strategy fits into her life, (2) recall what strategies or activities may have worked for her previously and specify how, (3) find a balance between doing too much and doing too little, and (4) identify family members, friends, and health care team members to support her plan. For example, one woman rediscovered strategies of restoring her energy that had previously been helpful and applied them to address her CRF: “Spend time reading, listening to music, sitting and contemplating - all of those activities have been energizing in the past and I will endeavor to incorporate them back into my life.” Another woman listed specific friends and family members whom she planned to ask for help cooking meals, accompanying her to medical appointments, and being an exercise partner.
After thinking through each of these considerations, the participant and her nurse interventionist engaged in an intense individualization process that usually occurred over several weeks. Message board posts over time demonstrated that participants’ original overarching goals remained the same, but the strategies were adjusted frequently based on participant feedback from ongoing implementation and assessment. This back-and-forth progression allowed participants to enact their symptom management plan, assess its effectiveness, and make necessary modifications. The process of identifying, confirming, testing, and evaluating different CRF strategies resulted in refined, specific and individualized strategies intended to eventually ensure participants achieve their goal.
Participants’ message boards reflected a high level of personal investment in their individualization process, evidenced by the level of specificity with which the strategies were described. Some women wrote using a high level of personal detail, clearly visualizing how they would enact each strategy (e.g. “I’m going to try to carry something in my purse to keep a symptom journal and take it out when I am in the doctor’s office”). While some participants’ strategies remained very general, this may reflect difficulty or reluctance on the part of some participants to take general strategies and individualize them to their own lives.
Discussion
This study reveals the process by which women with recurrent ovarian cancer, assisted by a nurse, create individualized, patient-centered goals and strategies to improve CRF and explores the content of those goals and strategies. By understanding this process of individualization, the benefits of goal-setting for improved symptom management can be realized.24,25 Given patients’ hesitancy to report CRF because of misperceptions that it is either unavoidable or will result in changes to their primary cancer treatment,26 individualization of goals and strategies such as those described here for CRF among women with recurrent ovarian cancer may address patient barriers to symptom management.
CRF is notoriously one of the hardest symptoms to manage. Even with evidence-based recommendations to understand and treat CRF from the National Comprehensive Cancer Network27 and the Oncology Nursing Society,28 it is up to the patient and her health care team to develop specific behavior changes that can improve energy for that individual patient. For example, moderate-intensity, multimodal exercise is an evidence-based way to improve fatigue,29,30 but still must be adapted to fit the individual patient’s physical abilities, preferences, and barriers to engaging in regular exercise.
The current study demonstrates that individualization of CRF goals and strategies provides patients with an opportunity to select general strategies and subsequently refine them to fit their own lifestyle and priorities. Satisfactorily individualizing strategies requires a woman to imagine how she can take a given strategy and make it fit into her life, often by rediscovering previous approaches that worked and by identifying people who could assist her. This process can be especially empowering for women with recurrent ovarian cancer who are likely to receive several lines of multimodal chemotherapy and so experience CRF over longer periods of time and at a higher level of severity. If individualization of symptom management goals and strategies allows these patients to gain better control over their CRF, then it may also improve their quality of life and sense of control over the impact of their cancer on their daily lives.
The SMART acronym has been discussed in a number of professional literatures including rehabilitation, occupational therapy, and psychology and has been found to be associated with better goal attainment.31,32 In short, patients are more likely to achieve their health-related goals if they know (S) specifically what they want to accomplish, (M) how they will measure and know that they have reached this goal, that it is (A) achievable and realistic given available resources and motivation, that it is (R) relevant to their life, and that (T) there is a timely deadline associated with the outcome. Training health care providers to write SMART goals can help them in turn to elicit patient needs and translate them into SMART goals and strategies.33
In the current analysis, nurse interventionists encouraged use of SMART goals, yet women primarily focused on how symptom management goals and strategies could help them achieve personally-meaningful activities and priorities, suggesting that specificity and measurability were not as important to them as were practicality and coherence with personal priorities. For example, even though some goals (e.g. “I can feel like I have my life back even though I am taking chemo”) are not specific or measurable, they speak to the values and motivations participants had for addressing their CRF. Rather than, or in addition to, increasing the level of difficulty of symptom management goals as has been suggested by some investigators,34,35 using the individualization process to create evidence-based strategies that match the woman’s unique situation, motivations, and goals may increase her investment, enhance her adherence to the chosen strategy, and ultimately lead to goal achievement. Indeed, such individualization is consistent with Sackett et al.’s definition of evidence-based medicine as “the integration of best research evidence with clinical expertise and patient values.”36
This study has several limitations. Participants’ goals, strategies, and processes of individualization represent the experiences of recurrent ovarian cancer survivors with CRF and may not directly parallel symptom management processes of other patient populations. Additionally, these data are unable to separate the unique contributions of the nurse and participant in individualizing the goals and strategies. Finally, our analyses did not evaluate the effectiveness of different types of strategies or level of individualization in achieving better symptom management for CRF or for other symptoms.
Conclusion
These findings reinforce for oncology nurses that patients are eager to participate in developing goals and strategies when they have a high level of investment in the outcome. Goals that reflect patients’ individual priorities and strategies designed to target patient-selected symptoms represent individualized, personally-meaningful means by which patients can achieve their goals of improving their symptom distress. The themes of participants’ goals and types of strategies reported in this study can inform the content and design of symptom management tools and can be used as a templates from which patients can begin the process of developing their own personally meaningful goals and related strategies.
Future research should address the need for individualizing broad symptom management strategies to fit patient’s needs and priorities. Although CRF has served as an exemplar in this secondary analysis, the effectiveness of such a process can be tested to determine if the process of individualizing goals and strategies leads to improved symptom management for severe, distressing, and poorly managed symptoms in any serious or chronic illness. Moreover, research should prospectively examine how goal and strategy selection may impact participant enactment and lead to improved symptom management.
Oncology nurses and health professionals can apply these findings as they assist patients in creating individualized goals and strategies for any symptom or health-related problem. Individualization occurs by helping patients select a personally-relevant goal, visualizing specific strategies that help the patient achieve the overall goal, and continually revisiting and revising the strategies to find those that are most feasible and effective in helping the patient achieve his or her goal. Nurses can ask patients to imagine what each strategy would “look like” on daily basis, recall previously successful strategies, identify specific people to support their plan, and find a balance between aggressively working to improve the problem and overextending themselves.
Providers might consider such an intense intervention to be overly time-consuming. However, if individualized goals and strategies prove to yield positive symptom management outcomes, the initial time investment on the part of both patient and provider is likely to be offset by gains to the patient and by avoidance of greater investment of professional effort over a longer time for less fruitful outcomes.
Acknowledgments
Funding: National Institute of Nursing Research/NIH: R01 NR010735 (H. D.)
National Institute of Nursing Research/NIH: T32 NR011972 (T. H. and J.A.)
Footnotes
Conflicts of Interest: The authors have no funding or conflicts of interest to disclose.
References
- 1.Bodenheimer T, Handley MA. Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ Couns. 2009;76(2):174–180. doi: 10.1016/j.pec.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Locke EA, Latham GP. Building a practically useful theory of goal setting and task motivation: a 35-year odyssey. Am Psychol. 2002;57(9):705–717. doi: 10.1037//0003-066x.57.9.705. [DOI] [PubMed] [Google Scholar]
- 3.Mann T, de Ridder D, Fujita K. Self-regulation of health behavior: social psychological approaches to goal setting and goal striving. Health Psychol 2013. 2013;32(5):487–498. doi: 10.1037/a0028533. [DOI] [PubMed] [Google Scholar]
- 4.Needham P, Newbury J. Goal setting as a measure of outcome in palliative care. Palliative Medicine. 2004;18(5):444–451. doi: 10.1191/0269216304pm904oa. [DOI] [PubMed] [Google Scholar]
- 5.Pearson ES. Goal setting as a health behavior change strategy in overweight and obese adults: a systematic literature review examining intervention components. Patient Ed Couns. 2012;87(1):32–42. doi: 10.1016/j.pec.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Siegert RJ, McPherson KM, Taylor WJ. Toward a cognitive-affective model of goal-setting in rehabilitation: is self-regulation theory a key step? Disabil Rehabil. 2004;26(20):1175–1183. doi: 10.1080/09638280410001724834. [DOI] [PubMed] [Google Scholar]
- 7.Schulman-Green DJ, Naik AD, Bradley EH, McCorkle R, Bogardus ST. Goal setting as a shared decision making strategy among clinicians and their older patients. Patient Educ Couns. 2006;63(1):145–151. doi: 10.1016/j.pec.2005.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue. Cancer. 2012;118(S8):2261–2269. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 9.Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120:425–432. doi: 10.1002/cncr.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mock V, Atkinson A, Barsevick A, et al. NCCN Practice Guidelines for Cancer-Related Fatigue. Oncology. 2000;14(11A):151–161. [PubMed] [Google Scholar]
- 11.International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) Official Guidelines for Coding and Reporting; [Accessed January 29, 2016]. Available from: http://www.cdc.gov/nchs/icd/icd10cm.htm. [Google Scholar]
- 12.Payne JK. The trajectory of fatigue in adult patients with breast and ovarian cancer receiving chemotherapy. Oncol Nurs Forum. 2002;29(9):1334–1340. doi: 10.1188/02.ONF.1334-1340. [DOI] [PubMed] [Google Scholar]
- 13.American Cancer Society. Cancer Facts and Figures 2016. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 14.Gardner GJ, Jewell EL. Current and future directions of clinical trials for ovarian cancer. Cancer Control. 2011;18(1):44–51. doi: 10.1177/107327481101800106. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong DK. Relapsed ovarian cancer: challenges and management strategies for a chronic disease. The Oncologist. 2002;1(7 Supp 5):20–8. doi: 10.1634/theoncologist.7-suppl_5-20. [DOI] [PubMed] [Google Scholar]
- 16.Baker F, Denniston M, Smith T, West MM. Adult cancer survivors: how are they faring? Cancer. 2005;104(S11):2565–2576. doi: 10.1002/cncr.21488. [DOI] [PubMed] [Google Scholar]
- 17.Donovan HS, Ward SE, Song MK, Heidrich SM, Gunnarsdottir S, Phillips CM. An update on the representational approach to patient education. J Nurs Scholarsh. 2007;39(3):259–265. doi: 10.1111/j.1547-5069.2007.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donovan HS, Ward SE, Sereika, et al. Web-based symptom management for women with recurrent ovarian cancer: a pilot randomized controlled trial of the WRITE symptoms intervention. J Pain Symptom Manage. 2014;47(2):218–230. doi: 10.1016/j.jpainsymman.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sereika S, Engberg S. Development of standardized sociodemographic and co-morbidity questionnaires. Sigma Theta Tau International: 17th International Nursing Research Congress Focusing on Evidence-Based Practice; Pittsburgh, PA. 2006. [Google Scholar]
- 20.Donovan HS, Ward S, Sherwood P, Serlin RC. Evaluation of the Symptom Representation Questionnaire (SRQ) for assessing cancer-related symptoms. J Pain Symptom Manage. 2008;35(3):242–257. doi: 10.1016/j.jpainsymman.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coffey A, Atkinson P. Making Sense of Qualitative Data: Complementary Research Strategies. Thousand Oaks, CA: Sage Publishing; 1996. Concepts and coding. [Google Scholar]
- 22.Sandelowski M. Focus on research methods - Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334–340. doi: 10.1002/1098-240x(200008)23:4<334::aid-nur9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.Donovan HS, Ward S. A representational approach to patient education. J Nurs Scholarsh. 2001;33(3):211–216. doi: 10.1111/j.1547-5069.2001.00211.x. [DOI] [PubMed] [Google Scholar]
- 24.Reif K, de Vries U, Petermann F, Görres S. A patient education program is effective in reducing cancer-related fatigue: a multi-centre randomised two-group waiting-list controlled intervention trial. Eur J Oncol Nurs. 2013;17(2):204–213. doi: 10.1016/j.ejon.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Walker ER, Wexler B, DiIorio C, Escoffery C, McCarty F, Yeager KA. Content and characteristics of goals created during a self-management intervention for people with epilepsy. J Neurosci Nurs. 2009;41(6):312–321. doi: 10.1097/jnn.0b013e3181b6bec5. [DOI] [PubMed] [Google Scholar]
- 26.Luthy C, Cedraschi C, Pugliesi A, et al. Patients’ views about causes and preferences for the management of cancer-related fatigue—a case for non-congruence with the physicians? Support Care Cancer. 2011;19(3):363–370. doi: 10.1007/s00520-010-0826-9. [DOI] [PubMed] [Google Scholar]
- 27.National Comprehensive Caner Network. NCCN Clinical Practice Guidelines in Oncology: Cancer-Related Fatigue. Fort Washington, PA: NCCN; 2011. [Google Scholar]
- 28.Mitchell SA, Beck SL, Eaton LH. Fatigue. In: Eaton LH, Tipton JM, editors. Putting Evidence into Practice. Pittsburgh, PA: Oncology Nursing Society; 2009. pp. 149–174. [Google Scholar]
- 29.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: A systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008;34(5):700–741. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]
- 30.Minton O, Richardson A, Sharpe M, Hotopf M, Stone PC. Psychostimulants for the management of cancer-related fatigue: a systematic review and meta-analysis. J Pain Symptom Manage. 2011;41(4):761–767. doi: 10.1016/j.jpainsymman.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Bovend’Eerdt TJH, Botell RE, Wade DT. Writing SMART rehabilitation goals and achieving goal attainment scaling: A practical guide. Clin Rehab. 2009;23:352–361. doi: 10.1177/0269215508101741. [DOI] [PubMed] [Google Scholar]
- 32.Mynors-Wallis L. Problem-solving treatment in general psychiatric practice. Adv Psych Treat. 2001;7(6):417–425. [Google Scholar]
- 33.Marsland E, Bowman J. An interactive education session and follow-up support as a strategy to improve clinicians’ goal-writing skills: a randomized controlled trial. J Eval Clin Pract. 2010;16(1):3–13. doi: 10.1111/j.1365-2753.2008.01104.x. [DOI] [PubMed] [Google Scholar]
- 34.Smith A, Ntoumanis N, Duda JL. Goal striving, goal attainment, and well-being: Adapting and testing the self-concordance model in sport. J Sport and Exerc Psychol. 2007;29(6):763–82. doi: 10.1123/jsep.29.6.763. [DOI] [PubMed] [Google Scholar]
- 35.Strecher VJ, Seijts GH, Kok GJ, et al. Goal setting as a strategy for health behavior change. Health Educ Q. 1995;22(2):190–200. doi: 10.1177/109019819502200207. [DOI] [PubMed] [Google Scholar]
- 36.Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: What it is and what it isn’t. BMJ. 1996;312(7023):71–2. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]