Figure 4.
Optogenetic Targeting of ECT2 to the Plasma Membrane Induces Cleavage Furrow Formation
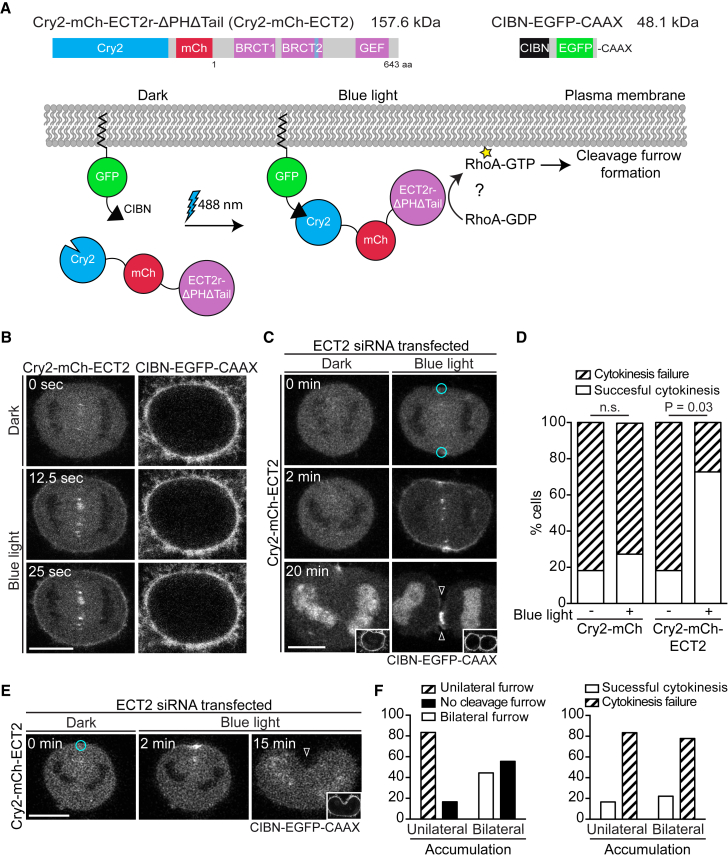
(A) Schematic depiction of optogenetic targeting of Cry2-mCh-ECT2 to the plasma membrane.
(B) Frames from confocal live-cell imaging. Cells were transfected with Cry2-mCh-ECT2 and CIBN-EGFP-CAAX. Cells were imaged 48 hr post-transfection, and the whole field was activated by scanning with a 488 nm laser at t = 0 s. Scale bars in this and the following panels represent 10 μm.
(C) Live-cell imaging with or without blue-light illumination. Cells stably expressing CIBN-EGFP-CAAX (inset) were transfected with Cry2-mCh-ECT2 and ECT2 siRNA and imaged 24 hr after siRNA transfection. Photoactivation was performed by illumination with a 488 nm laser within two small circular regions at the equatorial periphery, as marked in the image. Cleavage furrow ingression is indicated by open arrowheads.
(D) Quantification of cytokinetic phenotypes after optogenetic membrane targeting of ECT2 as described in (C). Metaphase or early anaphase cells with or without blue-light illumination were scored (n = 11, Fisher’s exact test).
(E) Live-cell imaging with unilateral blue-light illumination. Cells stably expressing CIBN-EGFP-CAAX (inset) were transfected with Cry2-mCh-ECT2 and ECT2 siRNA. Cells were imaged 24 hr after siRNA transfection. Photoactivation was induced by unilateral illumination with a 488 nm laser within the circular region at the equatorial periphery as indicated.
(F) Quantification of the furrow formation phenotype (left) and cytokinetic phenotype (right) in relation to protein distribution after unilateral membrane targeting of ECT2 as described in (E). Anaphase cells were scored (n = 15).
See also Figure S4.