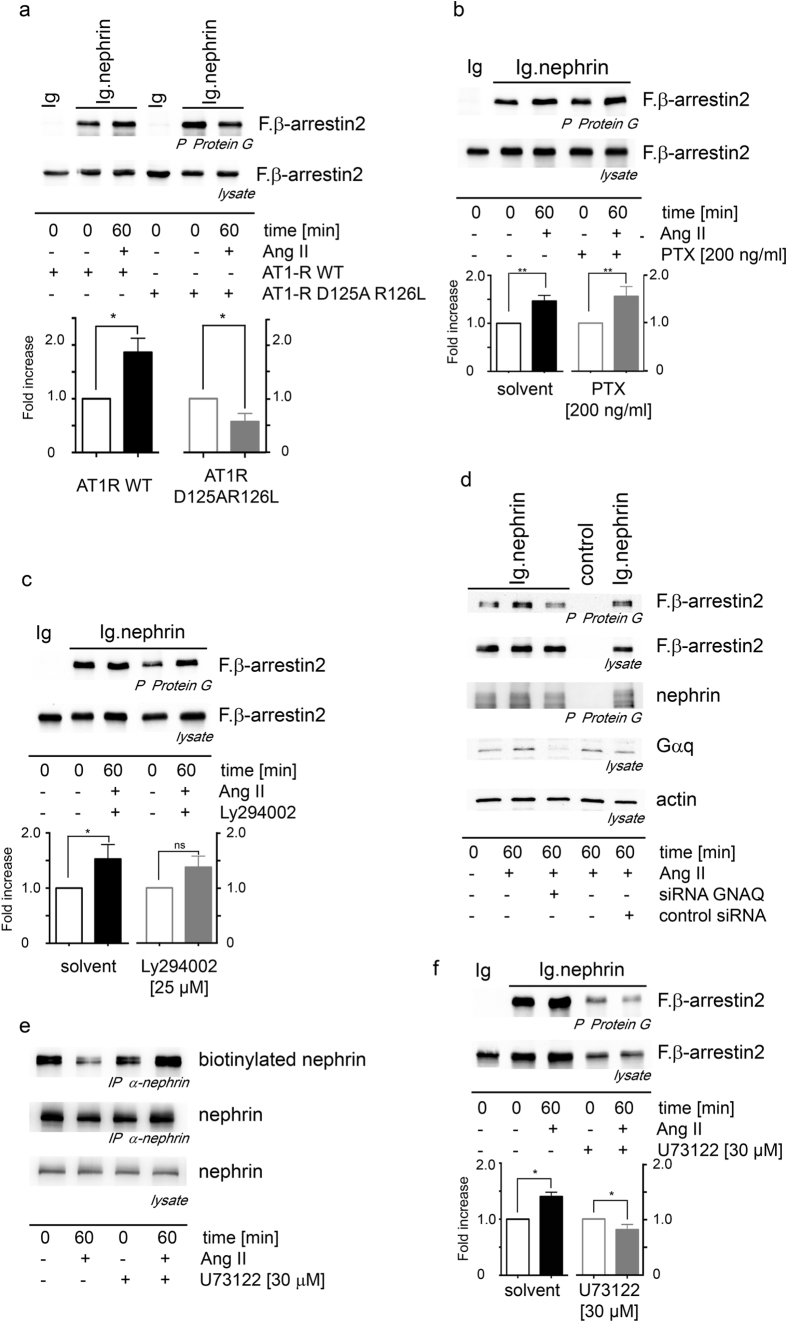
Figure 4. The Gαi signaling is essential in conveying the Ang II mediated, enhanced β-arrestin2 binding to the nephrin-c-terminus and nephrin endocytosis.
(a) The AT1 receptor D125AR126L mutant abolishes G-protein signaling and disables the Ang II induced enhanced β-arrestin2 binding to the nephrin-c-terminus (*n = 4, p = 0.014). (b) The inhibition of Gαi by pertussis toxin (PTX) does not prevent the Ang II mediated enhanced β-arrestin2 binding to the nephrin-c-terminus (**n = 5, p = 0.0079). (c) The inhibition of the PI3kinase with Ly294002 does not influence the Ang II enhanced interaction of the nephrin-c-terminus-β-arrestin2 excluding the Gβγ pathway (*n = 4, p = 0.0143, p = 0.15 for Ly294002). (d) Gαq mediates the AT1 receptor activation resulting in enhanced β-arrestin2 binding to the nephrin-c-terminus. The expression of GNAQ siRNA blocks the Ang II mediated enhancement of the β-arrestin2 binding to the nephrin-c-terminus. (e) The PLC inhibitor U73122 reduces the Ang II mediated nephrin endocytosis in HEK293T cells. (f) The PLC inhibitor U73122 abolishes the Ang II mediated enhanced β-arrestin2 binding to the nephrin-c-terminus in HEK293T (*n = 3, p = 0.0318). The use of the PLC inhibitor U73122 causes a reduced protein expression compared to conditions without U73122. All experiments were conducted in HEK293T cells. Comparable amounts of the nephrin-c-terminus fusion protein expression and its control were controlled by western blot. The expression of equivalent amounts of the AT1-receptors and its mutants was ensured by RT-PCR. The expression of the transfected nephrin fusion proteins and its control is shown in the supplemental Fig. 1Se–g. The expression of equivalent amounts of the AT1-receptors and its mutants was ensured by RT-PCR (supplemental Fig. 3Sb).