Abstract
Electrical stimulation (ES) is known to guide the development and regeneration of many tissues. However, although preclinical and clinical studies have demonstrated superior effects of ES on cartilage repair, the effects of ES on chondrogenesis remain elusive. Since mesenchyme stem cells (MSCs) have high therapeutic potential for cartilage regeneration, we investigated the actions of ES during chondrogenesis of MSCs. Herein, we demonstrate for the first time that ES enhances expression levels of chondrogenic markers, such as type II collagen, aggrecan, and Sox9, and decreases type I collagen levels, thereby inducing differentiation of MSCs into hyaline chondrogenic cells without the addition of exogenous growth factors. ES also induced MSC condensation and subsequent chondrogenesis by driving Ca2+/ATP oscillations, which are known to be essential for prechondrogenic condensation. In subsequent experiments, the effects of ES on ATP oscillations and chondrogenesis were dependent on extracellular ATP signaling via P2X4 receptors, and ES induced significant increases in TGF-β1 and BMP2 expression. However, the inhibition of TGF-β signaling blocked ES-driven condensation, whereas the inhibition of BMP signaling did not, indicating that TGF-β signaling but not BMP signaling mediates ES-driven condensation. These findings may contribute to the development of electrotherapeutic strategies for cartilage repair using MSCs.
Articular cartilage is a unique load-bearing tissue that lacks vascular, neural, and lymphatic tissues. Articular cartilage cannot spontaneously regenerate in vivo because chondral defects do not penetrate the subchondral bone and therefore cannot be accessed by blood supply or mesenchymal stem cells (MSCs) from bone marrow1,2. Hence, researchers and surgeons have developed various techniques to repair cartilage tissues3. However, most current cartilage repair techniques eventually lead to the formation of fibrocartilage and cartilage degeneration4. Accordingly, autologous chondrocyte implantation has been used for cartilage repair but is associated with several disadvantages, including limited cell sources and frequent injury of healthy cartilage during surgery, further encouraging formation of inferior fibrocartilage at defect sites5.
Owing to their capacity for self-renewal and differentiation into adipocytes, cartilage, bone, tendons, muscle, and skin, MSCs are an attractive cell source for cartilage defect therapies6,7,8,9,10,11. Furthermore, because MSCs are free of both ethical concerns and teratoma risks, MSCs have considerable therapeutic potential12. Therefore, it is important to develop effective and safe methods for the induction of MSC chondrogenesis and for the production of stable cartilaginous tissue by these cells. Multiple previous studies have demonstrated the effects of various chemical factors, such as soluble growth factors, chemokines, and morphogens, on chondrogenesis. In particular, transforming growth factors (TGF-β) and bone morphogenetic proteins (BMPs) have been shown to play essential roles in the induction of chondrogenesis13,14. Although these growth factors have great therapeutic potential for cartilage regeneration, growth factor-based therapies have several clinical complications, including high dose requirements, low half-life, protein instability, higher costs, and adverse effects15,16. Recent studies demonstrate that physical factors regulate cell differentiation and tissue development17,18,19,20,21, suggesting therapeutic potential of physical factors as alternatives to chemical agents for cartilage regeneration.
Endogenous electrical signals have been observed in articular cartilage during physiological processes, prompting the application of various electrical stimulation (ES) and electromagnetic field (EMF) inducers to in vitro chondrogenesis and in vivo cartilage repair22,23,24. In particular, studies using animal models show that ES and EMF improve healing of cartilage defects by increasing cell proliferation, glycosaminoglycan synthesis, and the expression of extracellular matrix genes, and by reducing the production of inflammatory mediators25,26,27,28,29. However, the precise roles of ES and EMF in cartilage repair remains unclear, and the effects of ES and EMF have only been observed in the surrounding cartilage, and not in articular defects. These observations indicate that ES or EMF alone have limited therapeutic efficacy for the repair of large osteochondral defects. Autologous chondrocyte transplants have been successfully used to repair large osteochondral defects30. Thus, multiple studies have investigated the effects of ES on proliferation and synthesis of cartilage extracellular matrix proteins in chondrocytes31,32,33,34. However, chondrocytes gradually decrease in number with age33, and it is difficult to obtain sufficient chondrocyte numbers to repair large defects due to limited life span and de-differentiation with downregulation of cartilage-marker genes during culture34,35. As an alternative, MSCs with self-renewing abilities can differentiate into chondrocytes and offer a reliable resource for ES-based therapies for damaged cartilage defects. However, although recent studies have reported the effects of ES on proliferation and differentiation of MSCs36,37, it remains unclear whether ES induces chondrogenic differentiation of MSCs.
Our previous studies demonstrated that intracellular ATP levels oscillate during chondrogenic differentiation and the ATP oscillations play critical roles in prechondrogenic condensation38,39, and that extracellular ATP signaling mediates the ATP oscillations during chondrogenesis40. ES has been shown to activate extracellular ATP signaling in a variety of cell types41,42,43. Therefore, we hypothesized that ES induces ATP oscillations in MSCs by stimulating extracellular ATP signaling and consequently drives MSC chondrogenesis. In the present study, we demonstrated that ES induces ATP oscillations and promotes MSC chondrogenesis in the absence of exogenous growth factors. Moreover, ES induced MSC chondrogenesis more effectively than treatments with chondrogenic medium (CM) supplemented with soluble growth factors. Accordingly, further experiments showed that extracellular ATP signaling via P2X4 receptors was responsible for ATP oscillations and mediated chondrogenesis following ES. In addition, ES-driven chondrogenesis depended on both TGF-β and BMP signaling pathways. However, TGF-β signaling, but not BMP signaling, was involved in ES-driven condensation. The present data suggest that ES has high potential as an MSC-based therapy for cartilage regeneration.
Results
ES induces calcium/ATP oscillations and MSC condensation
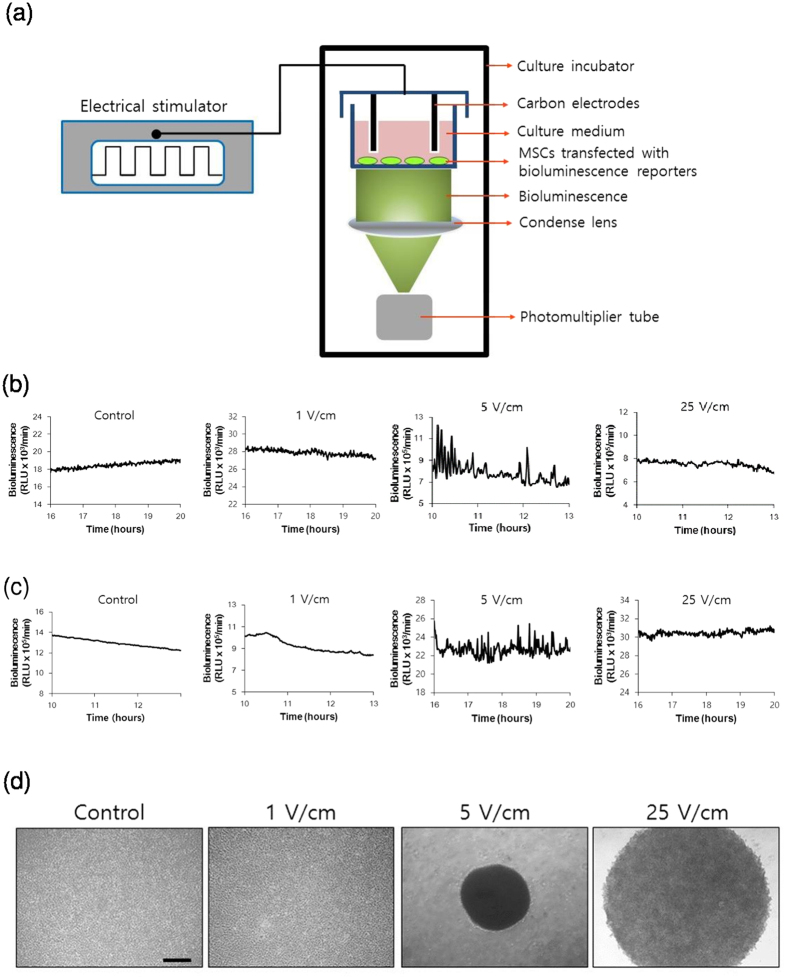
Flow cytometry analysis showed that the expanded MSCs were positive for typical MSC markers (Sca-1, CD44, CD73) but showed low expression of markers of hematopoietic stem cells (CD34), macrophages (CD11b), and granulocytes (CD45), which confirmed that the expanded MSCs exibited the characteristics of MSCs (Supplementary Fig. S1). To determine whether ES induces ATP oscillations in MSCs, we monitored temporal changes in intracellular ATP levels using a bioluminescent ATP-dependent luciferase (Luc) reporter gene fused to a constitutive ACTIN promoter (PACTIN-Luc). Following transfection of MSCs with PACTIN-Luc, bioluminescence intensity was measured in real-time during ES of 0, 1, 5 or 25 V/cm at 5 Hz (Fig. 1a). In these experiments, ES of 5 V/cm induced ATP oscillations of ~ 5 min periods, whereas ES of 0, 1, or 25 V/cm did not (Fig. 1b). Because ATP oscillations were driven by changes in Ca2+ concentrations during chondrogenesis38, we examined Ca2+ oscillations using the bioluminescent Ca2+ reporter Aequorin (AQ) gene fused to a CMV promoter (PCMV-AQ)44. ES of 5 V/cm consistently induced Ca2+ oscillations, whereas ES of 0, 1, or 25 V/cm did not (Fig. 1c), suggesting that optimized ES can drive fluctuations of both Ca2+ and ATP. We previously showed that growth factors such as TGF-βs and insulin induce prechondrogenic condensation by generating Ca2+/ATP oscillations39. Consistently, ES of 5 V/cm which induced Ca2+/ATP oscillations led to compact condensation of MSCs, whereas ES of 0, 1, or 25 V/cm did not (Fig. 1d). Moreover, time-course observations showed that ES of 5 V/cm induced gradual aggregation of MSCs into compact structures within 3 days, corresponding with the effects of chondrogenic medium (CM) supplemented with growth factors such as TGF-βs and insulin (Supplementary Fig. S2). These results indicate that ES induces prechondrogenic condensation by driving Ca2+/ATP oscillations, even in the absence of exogenous growth factors.
Figure 1. Electrical stimulation induces ATP/Ca2+ oscillations and MSC condensation.
(a) Schematic of the real-time bioluminescence monitoring system for ES treated MSCs transfected with bioluminescence reporters (b) Real-time monitoring of intracellular ATP levels in MSCs under ES (0, 1, 5, and 25 V/cm, 8 ms, 5 Hz) using a bioluminescence ATP reporter (PACTIN-Luc) (c) Real-time monitoring of intracellular Ca2+ levels under ES (0, 1, 5, and 25 V/cm, 8 ms, 5 Hz) using a bioluminescence Ca2+ reporter (PCMV-AQ) (d) Condensation behaviors of MSCs in micromass culture were examined using phase contrast images during culture for 3 days under ES (0, 1, 5, and 25 V/cm, 8 ms, 5 Hz); Scale bars, 500 μm.
ES induces MSC chondrogenesis
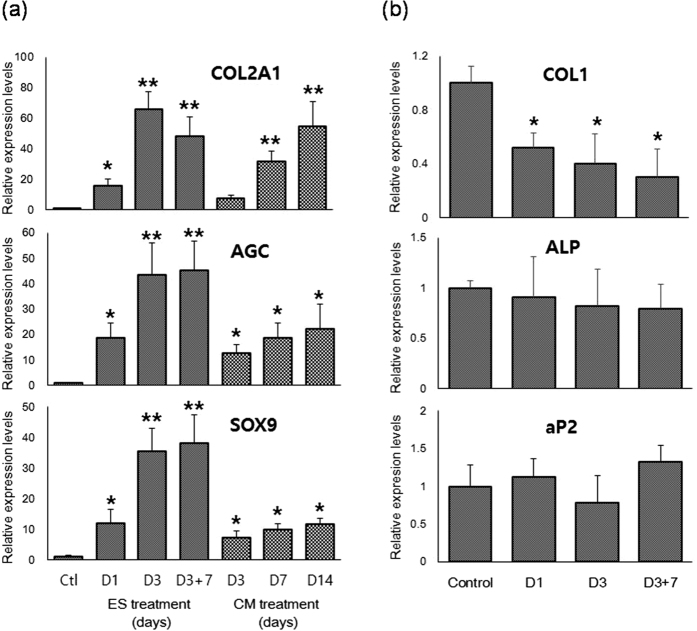
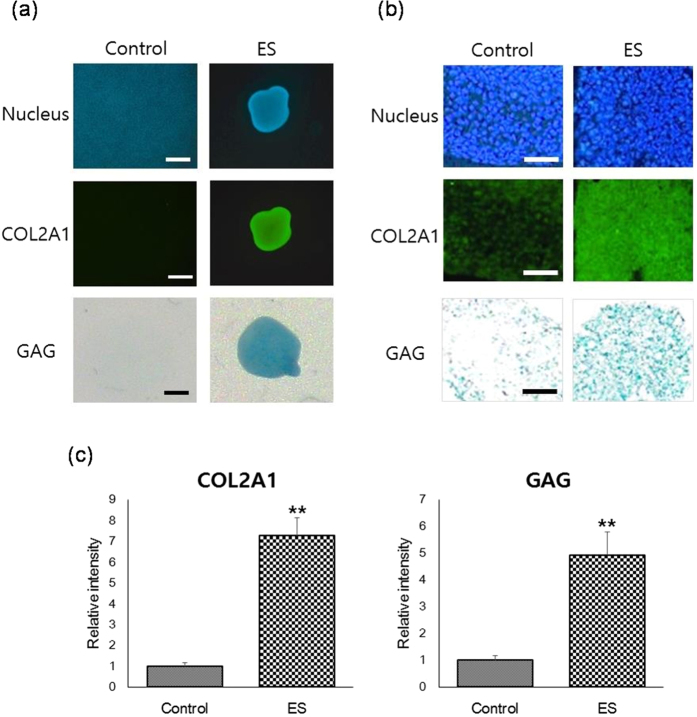
In further experiments, the effects of optimized ES on chondrogenic differentiation were examined. Since ES for 3 days had little effect on cell damage (<5%) but induced significant cell death (almost 50%) after 7 days (Supplementary Fig. S3), ES was performed for 3 days. Gene expression of chondrogenic markers such as type II collagen (COL2A1), aggrecan (AGC), and SRY (Sex Determining Region Y)-Box 9 (SOX9)45,46,47 was analyzed at 1 and 3-day of ES treatment and 7-day post-ES treatment. Quantitative real-time RT-PCR analyses showed that ES significantly enhanced gene expression of chondrogenic markers within 3 days of ES treatment, revealing a 66-fold increase in COL2A1, a 43-fold increase in AGC, and a 35-fold increase in SOX9 expression at 3-day of ES (Fig. 2a). Moreover, increases of chondrogenic marker expression in MSCs treated with ES for 3 days were much higher than those in the CM exposed cells, and were greater than or equal to expression levels in MCSs that were fully differentiated into chondrocytes following treatment with CM for 14 days (Fig. 2a). These data suggest that ES induces MSC chondrogenesis more effectively than CM. Moreover, it was found that the chondrogenic markers were highly expressed for as long as 7 days after the last ES treatment (Fig. 2a), which confirmed that chondrogenesis was induced in MSCs by ES. In addition, ES led to significant decreases in the expression of type I collagen (COL1; Fig. 2b). This result indicates that ES induces differentiation of MSCs into not fibrocartilaginous tissues but hyaline cartilaginous tissues48,49,50,51. In contrast, ES did not significantly change the expression of the osteogenic marker alkaline phosphatase (ALP), or the adipogenic marker adipocyte protein 2 (aP2; Fig. 2b), indicating that ES does not induce osteogenesis or adipogenesis. Additionally, immunostaining and alcian blue staining analyses showed significantly higher expression of type II collagen and GAGs in ES-treated MSCs compared with control cells (Fig. 3a–c). Taken together, these data suggest that ES induces MSC chondrogenesis for hyaline cartilage regeneration even in the absence of exogenous growth factors.
Figure 2. ES enhances mRNA expression of chondrogenic markers in MSCs.
Relative levels of mRNA were determined by quantitative real-time PCR in relation to beta-actin. (a) Gene expression of type II collagen (COL2A1), aggrecan (AGC), and Sox9 in MSCs was determined at various time points during ES treatment (5 V/cm, 8 ms, 5 Hz) and CM treatment. Data are presented as means ± standard deviations (S.D.). Statistical analyses were performed using ANOVA (Dunnett’s test); **p < 0.01, *p < 0.05 versus Ctl (control; MSCs cultured in maintenance medium for 3 days). (b) Gene expression of type 1 collagen (COL1), alkaline phosphatase (ALP), and adipocyte protein (aP2) was determined at various time points during ES treatment (5 V/cm, 8 ms, 5 Hz). Data show mean ± S.D. Statistical analyses were performed using ANOVA (Dunnett’s test); **p < 0.01, *p < 0.05 versus control (MSCs cultured in maintenance medium for 3 days).
Figure 3. ES enhances expression of COL2A1 and GAGs in MSCs.
(a) Immunofluorescent staining (COL2A1; green) and alcian blue staining (glycosaminoglycan (GAG); blue) of micromass cultures after culture in maintenance medium (control) or maintenance medium with ES (5 V/cm, 8 ms, 5 Hz) for 3 days (ES). Nuclei were stained blue using Hoechst 33342; Scale bars, 500 μm. (b) Immunofluorescent staining (COL2A1; green) and alcian blue staining (GAG; blue) of paraffin-embedded sections of micromass cultures after culture in maintenance medium (control) or maintenance medium with ES (5 V/cm, 8 ms, 5 Hz) for 3 days (ES). Nuclei were stained blue using Hoechst 33342; Scale bars, 100 μm. (c) Quantitative analysis of immunostaining intensity and alcian blue staining intensity. Data are presented as means ± S.D. and differences were identified using Students t-test, **p < 0.01.
Extracellular ATP signaling via P2X4 receptor mediates ATP oscillations, condensation, and subsequent chondrogenesis following ES
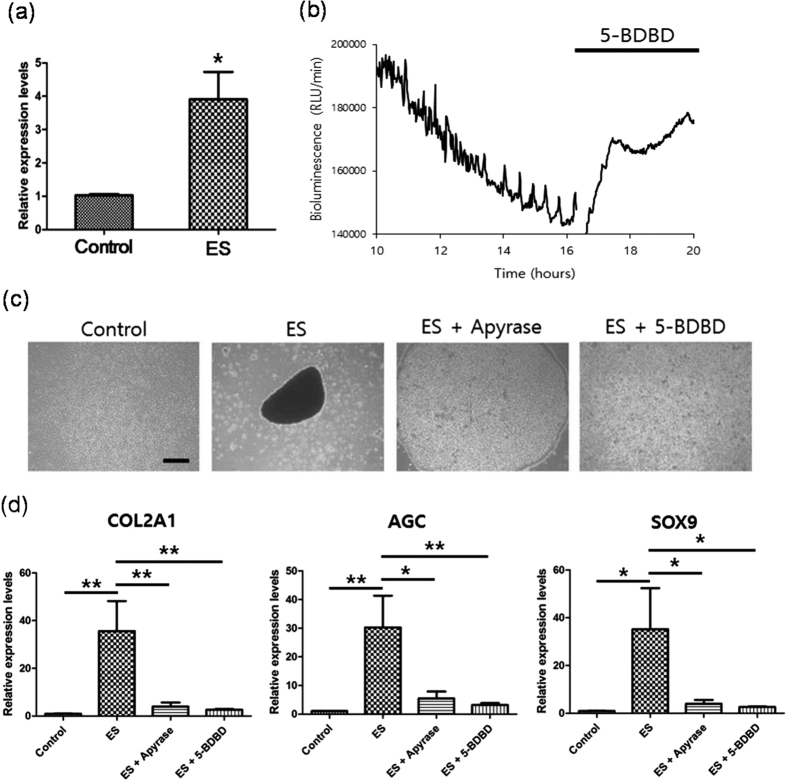
Extracellular ATP signaling via the P2X4 receptor reportedly plays key roles in prechondrogenic condensation by mediating ATP oscillations36. In the present study, ES enhanced the expression of P2X4 receptor mRNA in MSCs (Fig. 4a), suggesting that extracellular ATP signaling via the P2X4 receptor is involved in ATP oscillations, MSC condensation, and chondrogenesis following ES. In agreement, the P2X4 purinergic receptor inhibitor 5-BDBD inhibited ES-driven ATP oscillations (Fig. 4b). In subsequent experiments, it was examined whether extracellular ATP signaling via the P2X4 receptor was associated with ES-driven condensation and subsequent chondrogenesis. 5-BDBD almost completely inhibited ES-driven condensation, and apyrase significantly suppressed this process (Fig. 4c). In addition, ES did not enhance the expression of the chondrogenic markers COL2A1, AGC, and SOX9 in MSCs treated with either apyrase or 5-BDBD (Fig. 4d). Hence, extracellular ATP signaling via P2X4 receptors mediates ES-driven MSC condensation and chondrogenesis.
Figure 4. Extracellular ATP signaling via P2X4 receptors mediates ES-driven ATP oscillations, prechondrogenic condensation, and chondrogenesis.
(a) Real-time gene expression analyses of P2X4 receptors in micromass cultures of MSCs after culture for 3 days in maintenance medium (control) or maintenance medium under ES. Data are presented as means ± S.D. and differences were identified using Students t-test, *p < 0.05 (b) Effects of 5-BDBD on ES-driven ATP oscillations; MSCs in micromass culture were treated with 5-BDBD after induction of PACTIN-Luc oscillations by ES. Bioluminescence monitoring was performed after application of ES to MSCs (time = 0 h). (c) Effects of apyrase and 5-BDBD on ES-driven MSC condensation; MSCs in micromass culture were examined using phase contrast images after 3 days culture in maintenance medium (control), with ES (ES), with ES plus apyrase (ES + apyrase), or with ES plus 5-BDBD (ES + 5-BDBD); Scale bars, 500 μm. (d) Suppressive effects of apyrase and 5-BDBD on ES-induced type II collagen (COL2A1), aggrecan (AGC), and Sox9 expression. After 3 days culture in maintenance medium (control), with ES (ES), with ES plus apyrase (ES + apyrase), or with ES plus 5-BDBD (ES + 5-BDBD), gene expression was analyzed in MSCs using real-time PCR. Data are presented as means ± S.D. and differences were identified using ANOVA; **p < 0.01, *p < 0.05.
Intercellular communications mediates ES-driven chondrogenesis
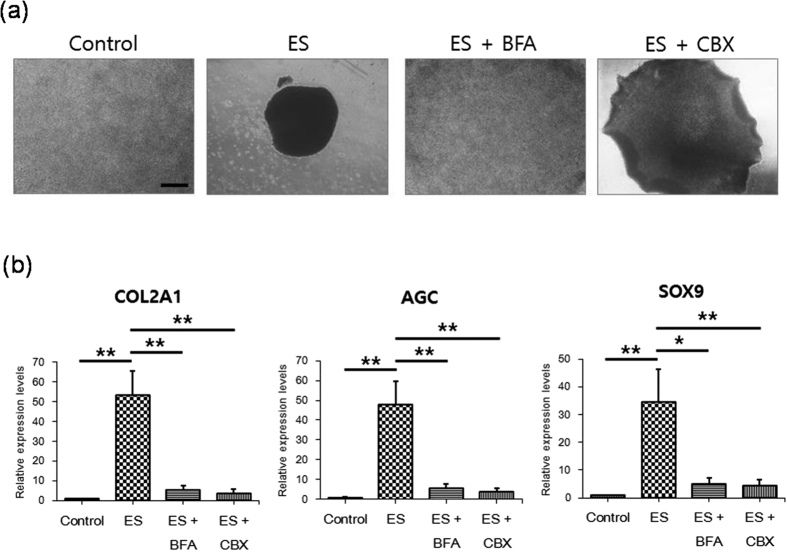
It was known that ES influences intercellular communications such as paracrine signaling52 and gap junction53. We found that BFA, which blocks classical secretion of paracrine factors, suppressed ES-driven condensation and ES-driven increases of COL2A1, AGC, and SOX9 expression (Fig. 5a,b). In addition, the gap-junction inhibitor carbenoxolone also suppressed ES-driven condensation and ES-driven increases of expression of the chondrogenic markers (Fig. 5a,b). This result indicates that ES induces chondrogenesis by activating the release of paracrine factors and the gap-junction activity.
Figure 5. Paracrine signals and gap junction mediates MSC condensation and chondrogenesis following ES.
(a) Effects of BFA and CBX on ES-driven MSC condensation; MSCs in micromass culture were examined using phase contrast images after 3 days culture in maintenance medium (control), with ES (ES), with ES plus BFA (ES + BFA), or with ES plus CBX (ES + CBX); Scale bars, 500 μm. (b) Suppressive effects of BFA and CBX on ES-induced type II collagen (COL2A1), aggrecan (AGC), and Sox9 expression. After 3 days culture in maintenance medium (control), with ES (ES), with ES plus BFA (ES + BFA), or with ES plus CBX (ES + CBX), gene expression was analyzed in MSCs using real-time PCR. Data are presented as means ± S.D. and differences were identified using ANOVA; **p < 0.01, *p < 0.05.
TGF-β signaling mediates MSC condensation and chondrogenesis following ES
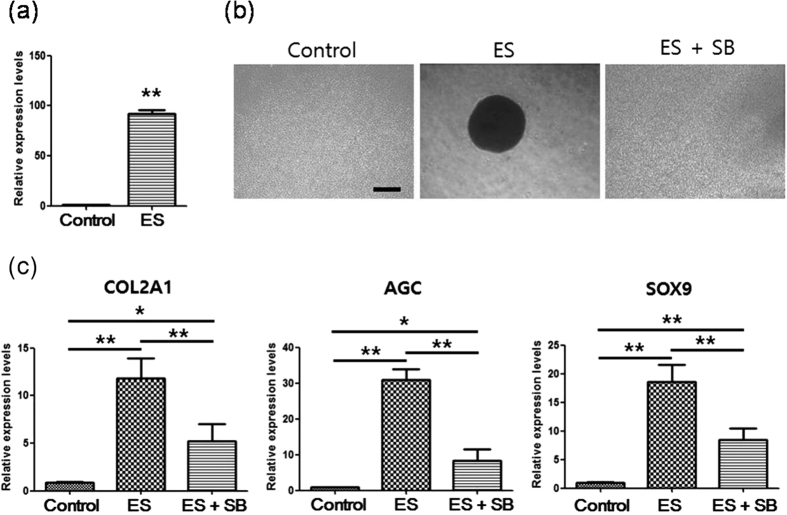
TGF-β signaling reportedly induces prechondrogenic condensation and chondrogenesis through ATP oscillations39. The present study showed that ES led to much higher mRNA expression of TGF-β1 (74 fold) than expression in control cells (Fig. 6a), suggesting that TGF-β signaling is involved in ES-driven MSC condensation and chondrogenesis. In agreement, inhibition of TGF-β signaling by SB-431542 almost completely blocked ES-driven condensation and significantly suppressed ES-driven increases of COL2A1, AGC, and SOX9 expression (Fig. 6b,c). Although these data indicate that ES induces MSC condensation and chondrogenesis by activating TGF-β signaling, SB-431542 did not completely suppress ES-driven induction of chondrogenic markers (Fig. 6c), suggesting that other growth factors and cytokines also mediate the actions of ES.
Figure 6. TGF-β signaling mediates MSC condensation and chondrogenesis following ES.
(a) Real-time gene expression analysis of TGF-β1 in micromass cultures of MSCs after culture for 3 days in maintenance medium (control) or with ES (ES); Data are presented as means ± S.D. and differences were identified using Students t-test, **p < 0.01 (b) Effects of SB-431542 (SB) on ES-driven MSC condensation; MSCs in micromass culture were examined using phase contrast images after 3 days culture in maintenance medium (control), with ES (ES), or with ES plus SB-431542 (ES + SB); Scale bars, 500 μm. (c) Effects of SB on ES-induced enhancement of type II collagen (COL2A1), aggrecan (AGC), and Sox9 expression; After 3 days culture in micromass cultures without treatment (control), with ES (ES), or with ES plus SB-431542 (ES + SB), gene expression was analyzed in MSCs using real-time PCR. Data are presented as means ± S.D. Differences were identified using ANOVA; **p < 0.01, *p < 0.05.
BMP signaling mediates ES-induced chondrogenesis, but not ES-induced condensation
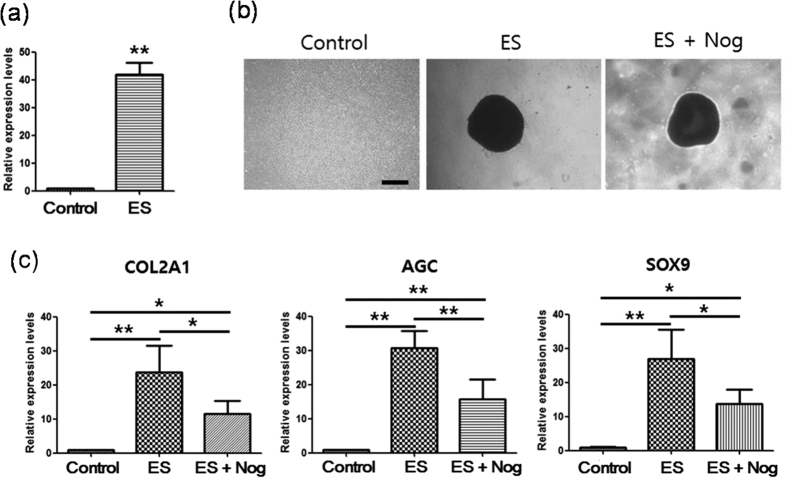
BMPs have been shown to play important roles in cartilage development54,55. Moreover, the present experiments showed that in comparison with non-treated controls, ES increased BMP2 expression by 42 fold (Fig. 7a). In addition, the inhibitor of BMP signaling noggin suppressed ES-driven increases in COL2A1, AGC, and SOX9 mRNA expression (Fig. 7c). However, noggin did not suppress ES-driven condensation (Fig. 7b), indicating that BMP signaling mediates ES-driven chondrogenesis, but not ES-driven condensation.
Figure 7. BMP signaling mediates ES-driven chondrogenesis, but not ES-driven condensation.
(a) Real-time gene expression analysis of BMP2 in micromass cultures of MSCs after 3 days in maintenance medium (control) or in maintenance medium with ES (ES). Data are presented as means ± S.D. and differences were identified using Students t-test, **p < 0.01 (b) Effect of noggin (Nog) on ES-induced MSC condensation; MSCs in micromass culture were examined using phase contrast images after 3 days culture in maintenance medium (control), with ES, or with Nog and ES; Scale bars, 500 μm. (c) Effect of Nog on ES-induced enhancement of type II collagen (COL2A1), aggrecan (AGC), and Sox9 expression. After 3 days micromass culture of MSCs with no treatment (control), with ES (ES), or with ES and Nog, gene expression was analyzed using real-time PCR. Data are presented as means ± S.D. Differences were identified using ANOVA; **p < 0.01, *p < 0.05.
Discussion
ES is a versatile treatment that remains poorly understood in the context of stem cell-based therapy. Herein, we demonstrate that ES significantly enhances the expression of chondrogenic markers (Figs 2a and 3a,b), but significantly decreases COL1 expression in MSCs (Fig. 2b). These data indicate that ES induces MSC differentiation into hyaline chondrogenic cells, and provide evidence of the potential of electrically stimulated MSCs to efficiently regenerate hyaline cartilage in the absence of additional exogenous chemical factors.
Our previous results demonstrated that ATP oscillations driven by chondrogenic growth factors such as TGF beta and insulin play essential roles for prechondrogenic condensation that is the initial step of chondrogenesis by inducing oscillatory expression of proteins involved in actin dynamics, cell migration, and adhesion which leads to collective migration and adhesion38,56. The present results demonstrate that ES generates Ca2+/ATP oscillations in MSCs even in the absence of exogenous growth factors (Fig. 1b). Since ES directly regulates voltage-gated Ca2+ channels57, ES can drive Ca2+ oscillations by modulating voltage-gated Ca2+ channels. In addition, since extracellular ATP signaling modulates Ca2+ flux by producing diacylglycerol and inositol 1,4,5-triphosphate, activating protein kinase C, and by mobilizing intracellular Ca2+ in multiple cell types58, ES can induces Ca2+ oscillation by extracellular ATP signaling via the P2X4 receptor, which is supported by the present result that P2X4 ATP signaling mediates the actions of ES (Fig. 4). Increased Ca2+ levels activate ATP-consuming processes such as ion pumping and exocytosis59, decrease glucose consumption by inhibiting glycolytic enzymes60, and decrease mitochondrial ATP production by abolishing mitochondrial membrane potential61, indicating the negative effects of Ca2+ on ATP levels. Accordingly, Ca2+ oscillations can drive ATP oscillations. In addition, previous studies demonstrated that pulsed electrical fields or pulsed electromagnetic fields modulate cAMP levels by activating adenosine receptors such as A2A, A2b, and A3 receptors, which leads to activation of anti-inflammatory pathways and cellular proliferation in cartilage62,63,64,65. Our previous results showed that ATP oscillations are dependent on cAMP dynamics38,40. These results suggest that ES drive ATP oscillations by modulating cAMP levels.
We demonstrated that pharmacological inhibition of P2X4-mediated ATP oscillations suppressed ES-driven condensation (Fig. 4b,c). Previous study showed that Ca2+/ATP oscillations induced synchronized secretion of adhesion molecules and prechondrogenic condensation38. In agreement, extracellular ATP signaling reportedly mediates chemotaxis and morphological changes from spread to spherical shapes, and Ca2+ oscillations play critical roles in cell-cell communications that lead to platelet aggregation66,67,68. Hence, ES may induce synchronized secretion of adhesion molecules and paracrine signaling, cell migration, and spherical morphogenesis by activating extracellular ATP signaling and Ca2+/ATP oscillations, leading to prechondrogenic condensation.
In the present study, ES induced chondrogenesis by stimulating both TGF-β and BMP signaling (Figs 6 and 7)69,70,71. It was known that TGF-β signaling reportedly stimulated prechondrogenic condensation by inducing the production of fibronectin and N-cadherin, and subsequently enhanced the expression of chondrogenic markers in various in vitro models69,70, and BMPs also promote chondrogenesis and regulate formation of cartilage elements in the limb71. Moreover, BMP signaling was shown to enhances TGF-β-induced chondrogenesis72. In addition, ES activates voltage-sensitive sodium and calcium ion channels to induce Ca2+ influx57. Hence, because Ca2+ influx activates exocytotic secretion73, increased Ca2+ influx following ES may enhance secretion of TGF-βs and BMPs, likely contributing significantly to the induction of MSC chondrogenesis. These facts can explain why ES led to stronger and more rapid induction of chondrogenesis than CM supplemented with TGF-β1 (Fig. 2a).
Many studies have shown that TGF-β signaling precedes BMP signaling and effectively initiates MSC condensation, leading to increases in the size and numbers of MSC aggregates, while BMP signaling is more effective in aggregated MSCs than in low density MSCs and increases sizes but not numbers of MSC aggregates69,70,71,74. We also previously demonstrated that TGF-β signaling but not BMP signaling drives ATP oscillations, leading to prechondrogenic condensation39. These data suggest differential effects of TGF-β and BMP signaling pathways on chondrogenesis. Consistent with these results, the present result showed that pharmacological inhibition of TGF-β signaling suppressed ES-driven condensation (Fig. 6b), whereas inhibition of BMP signaling did not (Fig. 7b), indicating that ES-driven condensation is mediated by TGF-β signaling, but is not mediated by BMP signaling. TGF-β signaling has been shown to enhance extracellular ATP levels and thus activate extracellular ATP signaling75. Accordingly, TGF-β signaling is stimulated by ES and then activates P2X4 signaling to consequently induce MSC condensation, which suggests that P2X4 signaling mediates the differential effects between TGF-β and BMP signaling on chondrogenesis.
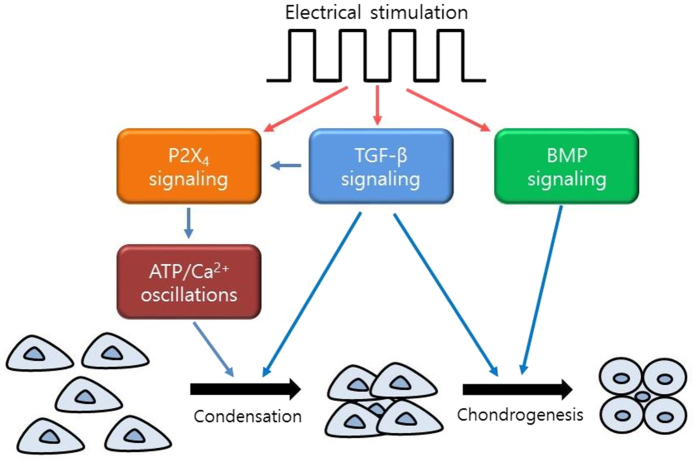
Based upon the findings from previous studies and the present study, the actions of ES for MSC chondrogenesis could be proposed: ES drives ATP/Ca2+ oscillations, leading to MSC condensation through TGF-β signaling and P2X4 signaling, and subsequently induces chondrogenesis through TGF-β signaling, BMP signaling and P2X4 signaling (Fig. 8).
Figure 8. Proposed model of the functions of electrotransduction for MSC chondrogenesis.
ES drives ATP/Ca2+ oscillations, leading to MSC condensation through TGF-β signaling and P2X4 signaling, and subsequently induces chondrogenesis through TGF-β signaling, BMP signaling and P2X4 signaling.
In summary, in this paper we demonstrate for the first time that ES drives Ca2+/ATP oscillations, leading to MSC chondrogenesis in the absence of exogenous cytokine or growth factor supplements, and optimized ES regimes for induction of MSC chondrogenesis. Subsequently, we showed that P2X4 signaling mediates ES-driven ATP oscillations and chondrogenesis, and TGF-β and BMP signaling both mediates ES-driven chondrogenesis but have differential effects on ES-driven condensation. These data will facilitate the development of a novel ES-based technology for cell therapy and ES-based rehabilitation for cartilage repair. However, further studies are required to establish ES-based therapeutic strategies with the potential to overcome limitations of cartilage repair.
Methods
Cell culture and light microscopy observations
Mouse MSCs which were produced from bone marrow that was isolated from C57BL/6 mice were purchased from Invitrogen (Carlsbad, CA, USA), and were expanded in Dulbecco’s Modified Eagle’s Medium/Ham’s Nutrient Mixture F-12 (DMEM/F12; Sigma-Aldrich, St. Louis, MO, USA) with GlutaMAX-I supplemented with 10% fetal bovine serum (FBS; Invitrogen). All experiments were performed in micromass cultures. Briefly, the expanded MSCs (passage 3–7) were harvested and resuspended in maintenance medium at 2 × 107 cells/ml. Droplets (10 μL) were carefully placed in each dish and cells were allowed to adhere at 37 °C for 1 h. Subsequently, 3 mL of maintenance medium were added to control and ES groups, while 3 mL of chondrogenic medium (CM; DMEM/F12, 1% ITS (Sigma-Aldrich), 10-ng/ml TGF-β1 (Peprotech, Rocky Hill, NJ, USA), 0.9-mM sodium pyruvate (Sigma-Aldrich), 50-μg/ml l-ascorbic acid-2-phosphate (Sigma-Aldrich), 10−7-M dexamethasone (Sigma–Aldrich), and 40-μg/ml l-proline (Sigma-Aldrich)) were added to CM group. To investigate the effects of chemical compounds on MSCs, culture medium was replaced with medium supplemented with 100-unit/ml apyrase (Sigma-Aldrich), which catalyzes the hydrolysis of ATP to AMP and inorganic phosphate, 100-μM 5-(3-bromophenyl)-1,3-dihydro-2Hbenzofuro[3,2-e]-1,4-diazepin-2-one (5-BDBD; Tocris Bioscience, Bristol, United Kingdom), which is an inhibitor of P2X4 purinergic receptors, 100 ng/ml noggin (R&D Systems), which is a BMP-specific antagonist protein, 10-μM SB-431542 (Sigma–Aldrich), which is an inhibitor of TGF-beta type I receptor, 100ng/ml brefeldin A (BFA), which is a inhibitor for protein secretion, and 100-μM carbenoxolone, which is a gap junction inhibitor. After 3, 7, and 14 days culture, microscope observations were performed using a phase contrast microscope (Nikon, Tokyo, Japan).
Flow cytometry
The cell surface markers of MSCs were analyzed using a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA, USA). Briefly, cells that reached 90% confluence were harvested using 0.25% EDTA and washed twice in Dulbecco’s phosphate buffered saline supplemented with 10% FBS. The cells for detecting CD11b, CD34, CD45, Sca-1, CD44 and CD73 were labeled directly with BB515 or PE-conjugated CD markers (rat anti-mouse CD11b [1: 100, BD Pharmingen; BD Biosciences, Franklin Lake, NJ, USA], rat anti-mouse CD34 [1: 100, BD Pharmingen], rat anti-mouse CD45 [1: 100, BD Pharmingen], rat anti-mouse Sca-1 [1: 100, BD Pharmingen], rat anti-mouse CD44 [1: 100, BD Pharmingen], rat anti-mouse CD73 [1: 100, BD Pharmingen]).
Electric stimulation
ES was applied to MSCs using a C-Pace EP culture pacer (IonOptix, MA, USA), which is a multi-channel stimulator designed for chronic stimulation of bulk quantities of cells in culture. This instrument emits bipolar pulses to culture media immersed carbon electrodes of a C-dish. ES was applied to MSCs cultured under conditions of high-density micromass (2 × 107 cells/ml) under electrical fields of 0, 1, 5, or 25 V/cm, with a duration of 8 ms and a frequency of 5.0 Hz. At indicated time points, MSCs were harvested in Trizol (Invitrogen) for real-time PCR analyses or were fixed using paraformaldehyde in phosphate-buffered saline (pH 7.4) for immunocytochemical analyses and alcian blue staining.
Transfection of cells with reporter genes and bioluminescence monitoring
For real-time monitoring of intracellular ATP levels in MSCs, MSCs were transfected with a bioluminescent luciferase reporter gene (Luc) fused to an ACTIN promoter (PACTIN-Luc) using Lipofectamine LTX (Invitrogen) and then the medium was replaced with recording medium (DMEM/F12 containing 10% FBS, 0.1-mM luciferin (Wako, Osaka, Japan), and 50-mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-NaOH (pH = 7.0)). For real-time monitoring of intracellular Ca2+ levels in MSCs, MSCs were transfected with a aequorin gene (AQ) fused to an CMV promoter (PCMV-AQ) using Lipofectamine LTX (Invitrogen) and then the medium was replaced with recording medium (DMEM/F12 containing 10% FBS, 5-μM coelenterazine (Invitrogen), and 50-mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-NaOH (pH = 7.0)). Bioluminescence intensity was continuously measured using a dish-type luminescence detector (Kronos; ATTO, Osaka, Japan) at 1 min intervals under ES.
Lactate Dehydrogenase (LDH) Release Assays
LDH release assays were performed to assess the cytotoxicity of ES using LDH-cytotoxicity assay kits (DoGen, Korea) according to the manufacturer’s instructions. After ES for 3 or 7 days, supernatants from each dish were transferred to fresh, flat bottom 96-well culture plates containing 100-μL reaction mixtures, and were incubated for 30 min at room temperature. Formazan absorbance was then measured at 480 nm using a microplate reader (TECAN, Switzerland).
Real-time PCR analysis
Total RNA was isolated from various MSCs cultures using the Direct-zol™ RNA MiniPrep (Zymo Research Corporation, Irvine, CA, U.S.A.) according to the manufacturer’s protocol. RNA concentrations were determined using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA), and reverse transcription reactions were performed using 0.2 μg of total RNA with a TOPscriptTM cDNA synthesis kit (enzynomics, Daejeon, Korea). The real-time PCRs for beta-actin, collagen II, and aggrecan were performed using the TOPrealTM qPCR 2X Pre MIX (enzynomics). Primer sequences are listed in Table 1. Real-time PCRs were performed using a StepOnePlus™ instrument (Applied Biosystems, Grand Island, NY, USA) at 95 °C for 15 min followed by 40 cycles of denaturation at 95 °C for 10 s, extension at 60 °C for 15 s, and annealing at 72 °C for 15 s. Gene expression levels were normalized to that of beta-actin and relative gene expression was calculated using the ddCT method.
Table 1. The primer sequences for Real-time PCR analysis.
| Gene | Forward primers | Reverse primers | Accession No. |
|---|---|---|---|
| COL2A1 | AGGGCAACAGCAGGTTCACATAC | TGTCCACACCAAATTCCTGTTCA | NM031163 |
| Aggrecan | AGTGGATCGGTCTGAATGACAGG | AGAAGTTGTCAGGCTGGTTTGGA | NM007424 |
| SOX9 | GAGGCCACGGAACAGACTCA | CTTCAGATCAACTTTGCCAGCTT | NM011448 |
| P2X4 | AGACGGACCAGTGATGCCTAAC | TGGAGTGGAGACCGAGTGAGA | NM011026 |
| TGF-β1 | GCTTCAGACAGAAACTCACT | GAACACTACTACATGCCATTAT | BC013738 |
| BMP2 | ACTTTTCTCGTTTGTGGAGC | GAACCCAGGTGTCTCCAAGA | NM007553 |
| ALP | CCAACTCTTTTGTGCCAGAGA | GGCTACATTGGTGTTGAGCTTT | NM007431 |
| COL1 | GCTCCTCTTAGGGGCCACT | CCACGTCTCACCATTGGG | NM007742 |
| aP2 | GTGTGATGCCTTTGTGGGAAC | CCTGTCGTCTGCGGTGATT | NM024406 |
| β-actin | AGGTCATCACTATTGGCAACGA | ATGGATGCCACAGGATTCCA | NM007393 |
Immunofluorescence staining and alcian blue staining
MSCs were fixed in 4% paraformaldehyde for 20 min at room temperature and were washed three times in phosphate buffered saline (PBS). Some samples were dehydrated through a graded ethanol series, infiltrated with xylene, embedded in paraffin, and sectioned at a thickness of 7-μm. After blocking in PBS containing 5% goat serum and 0.3% Triton X-100 for 60 min at room temperature, cells were incubated with rabbit anti-type II collagen antibody (1:500; EnoGene Biotech, New York, NY, USA) at 4 °C overnight, were washed three times in PBS containing 0.1% Triton X-100, and were then incubated with Alexa488-conjugated secondary antibody (1:200; Invitrogen) for 60 min at room temperature in the dark. Subsequently, cells were washed three times in PBS containing 0.1% Triton X-100 and nuclei were stained with Hoechst 33258 (Dojindo, Tokyo, Japan). To visualize accumulation of sulfated glycosaminoglycans (GAGs), cells were rinsed with PBS, fixed in paraformaldehyde for 20 min, stained with Alcian Blue Solution (pH 2.5; Nacalai tesque, INC, Japan) overnight at room temperature, and were then rinsed with distilled water three times. Accumulations of glycosaminoglycans were captured using a digital camera (Olympus, Tokyo, Japan). Expression levels of type II collagen and GAGs were quantified using immunofluorescence and alcian blue intensity profiles with the NIH IMAGE J program, and data were transferred into Microsoft Excel for further analyses.
Statistical analysis
The results are presented as means ± SD for all samples. The statistical differences between groups were analyzed by Students t-test, and multiple comparisons were performed by Fisher’s protected least significant difference (PLSD) or Dunnett’s test. A value of p < 0.05 was considered to indicate statistical significance.
Additional Information
How to cite this article: Kwon, H. J. et al. Electrical stimulation drives chondrogenesis of mesenchymal stem cells in the absence of exogenous growth factors. Sci. Rep. 6, 39302; doi: 10.1038/srep39302 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by Basic Science Research Program (2014R1A1A1002054) and Global Frontier Project (NRF-2013M3A6A4046061) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning.
Footnotes
Author Contributions H.J.K. and H.G.C. designed research; H.J.K. and G.S.L. performed research; H.J.K. and H.G.C. analyzed data; H.J.K. and H.G.C. wrote the paper. All authors discussed the results and commented on the paper.
References
- O’Driscoll S. W. The healing and regeneration of articular cartilage. J Bone Joint Surg Am 80, 1795 (1998). [PubMed] [Google Scholar]
- Mandelbaum B. R. et al. Articular cartilage lesions of the knee. Am J Sports Med 26, 853 (1998). [DOI] [PubMed] [Google Scholar]
- Smith G. D., Knutsen G. & Richardson J. B. A clinical review of cartilage repair techniques. J Bone Joint Surg Br 87, 445 (2005). [DOI] [PubMed] [Google Scholar]
- Kuroda R. et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage 15, 226 (2007). [DOI] [PubMed] [Google Scholar]
- Wood J. J. et al. Autologous cultured chondrocytes: adverse events reported to the United States Food and Drug Administration. J Bone Joint Surg Am 88, 503 (2006). [DOI] [PubMed] [Google Scholar]
- Chen Y., Shao J. Z., Xiang L. X., Dong X. J. & Zhang G. R. Mesenchymal stem cells: a promising candidate in regenerative medicine. Int J Biochem Cell Biol 40, 815 (2008). [DOI] [PubMed] [Google Scholar]
- Pittenger M., Vanguri P., Simonetti D. & Young R. Adult mesenchymal stem cells: potential for muscle and tendon regeneration and use in gene therapy. J Musculoskelet Neuronal Interact 2, 309 (2002). [PubMed] [Google Scholar]
- Makino S. et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest 103, 697 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney D. G. & Isakova I. Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr Pharm Des 11, 1255 (2005). [DOI] [PubMed] [Google Scholar]
- Tropel P. et al. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells 24, 2868 (2006). [DOI] [PubMed] [Google Scholar]
- Bhatia R. & Hare J. M. Mesenchymal stem cells: future source for reparative medicine. Congest Heart Fail 11, 87 (2005). [DOI] [PubMed] [Google Scholar]
- Mueller M. B. & Tuan R. S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 58(5), 1377–88 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuli R. et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 278(42), 41227–36 (2003). [DOI] [PubMed] [Google Scholar]
- Haas A. R. & Tuan R. S. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: II. Stimulation by bone morphogenetic protein-2 requires modulation of N-cadherin expression and function. Differentiation. 64, 77–89 (1999). [DOI] [PubMed] [Google Scholar]
- Lee S. H. & Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev 59, 339 (2007). [DOI] [PubMed] [Google Scholar]
- Seeherman H. & Wozney J. M. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytok Growth Fact Rev 16, 329 (2005). [DOI] [PubMed] [Google Scholar]
- Pouille P. A., Ahmadi P., Brunet A. C. & Farge E. Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci Signal 2, ra16 (2009). [DOI] [PubMed] [Google Scholar]
- Martin A. C., Kaschube M. & Wieschaus E. F. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg M. et al. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol 10, 429 (2008). [DOI] [PubMed] [Google Scholar]
- Rauzi M., Verant P., Lecuit T. & Lenne P. F. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol 10, 1401 (2008). [DOI] [PubMed] [Google Scholar]
- Desprat N., Supatto W., Pouille P. A., Beaurepaire E. & Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell 15, 470 (2008). [DOI] [PubMed] [Google Scholar]
- Grodzinsky A. J., Lipshitz H. & Glimcher M. J. Electromechanical properties of articular cartilage during compression and stress relaxation. Nature 275, 448 (1978). [DOI] [PubMed] [Google Scholar]
- Schmidt-Rohlfing B., Schneider U., Goost H. & Silny J. Mechanically induced electrical potentials of articular cartilage. J Biomech 35, 475 (2002). [DOI] [PubMed] [Google Scholar]
- Bassett C. A. & Pawluk R. J. Electrical behavior of cartilage during loading. Science 178, 982 (1972). [DOI] [PubMed] [Google Scholar]
- Baker B., Becker R. O. & Spadaro J. A study of electrochemical enhancement of articular cartilage repair. Clin Orthop Relat Res 102, 251 (1974). [DOI] [PubMed] [Google Scholar]
- Baker B., Spadaro J., Marino A. & Becker R. O. Electrical stimulation of articular cartilage regeneration. Ann N Y Acad Sci 238, 491 (1974). [DOI] [PubMed] [Google Scholar]
- Lippiello L., Chakkalakal D. & Connolly J. F. Pulsing direct current-induced repair of articular cartilage in rabbit osteochondral defects. J Orthop Res 8, 266 (1990). [DOI] [PubMed] [Google Scholar]
- Aaron R. K., Wang S. & Ciombor D. K. Upregulation of Basal TGFbeta1 Levels by EMF Coincident with Chondrogenesis: Implications for Skeletal Repair and Tissue Engineering. Journal of Orthopaedic Research 20, 233–240 (2002). [DOI] [PubMed] [Google Scholar]
- Ciombor D. M., Lester G., Aaron R. K., Neame P. & Caterson B. Low frequency EMF regulates chondrocyte differentiation and expression of matrix proteins. J Orthop Res 20(1), 40–50 (2002). [DOI] [PubMed] [Google Scholar]
- Brittberg M., Peterson L., Sjögren-Jansson E., Tallheden T. & Lindahl A. Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J Bone Joint Surg Am 85-A(Suppl 3), 109 (2003). [DOI] [PubMed] [Google Scholar]
- Armstrong P. F., Brighton C. T. & Star A. M. Capacitively coupled electrical stimulation of bovine growth plate chondrocytes grown in pellet form. J Orthop Res 6, 265 (1988). [DOI] [PubMed] [Google Scholar]
- Brighton C. T., Unger A. S. & Stambough J. L. In vitro growth of bovine articular cartilage chondrocytes in various capacitively coupled electrical fields. J Orthop Res 2, 15 (1984). [DOI] [PubMed] [Google Scholar]
- Wang W., Wang Z., Zhang G., Clark C. C. & Brighton C. T. Up-regulation of chondrocyte matrix genes and products by electric fields. Clin Orthop Relat Res 427, S163 (2004). [DOI] [PubMed] [Google Scholar]
- Ciombor D. M., Aaron R. K., Wang S. & Simon B. Modification of osteoarthritis by pulsed electromagnetic field–a morphological study. Osteoarthritis Cartilage 11, 455 (2003). [DOI] [PubMed] [Google Scholar]
- Goggs R., Carter S. D., Schulze-Tanzil G., Shakibaei M. & Mobasheri A. Apoptosis and the loss of chondrocyte survival signals contribute to articular cartilage degradation in osteoarthritis. Vet J 166, 140 (2003). [DOI] [PubMed] [Google Scholar]
- Hernández-Bule M. L., Paíno C. L., Trillo M. Á. & Úbeda A. Electric Stimulation at 448 kHz Promotes Proliferation of Human Mesenchymal Stem Cells. Cell Physiol Biochem 34, 1741–1755 (2014). [DOI] [PubMed] [Google Scholar]
- Hardy J. G. et al. Electrical stimulation of human mesenchymal stem cells on biomineralized conducting polymers enhances their differentiation towards osteogenic outcomes. J. Mater. Chem. B 3, 8059–8064 (2015). [DOI] [PubMed] [Google Scholar]
- Kwon H. J. et al. Synchronized ATP oscillations have a critical role in prechondrogenic condensation during chondrogenesis. Cell Death Dis 3, e278 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H. J. TGF-β but not BMP signaling induces prechondrogenic condensation through ATP oscillations during chondrogenesis. Biochem Biophys Res Commun 424, 793 (2012). [DOI] [PubMed] [Google Scholar]
- Kwon H. J. Extracellular ATP signaling via P2X(4) receptor and cAMP/PKA signaling mediate ATP oscillations essential for prechondrogenic condensation. J Endocrinol 214, 337 (2012). [DOI] [PubMed] [Google Scholar]
- Osorio-Fuentealba C. et al. Electrical stimuli release ATP to increase GLUT4 translocation and glucose uptake via PI3Kγ-Akt-AS160 in skeletal muscle cells. Diabetes 62, 1519 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M. & Attwell D. Non-synaptic release of ATP by electrical stimulation in slices of rat hippocampus, cerebellum and habenula. Eur J Neurosci 8, 1510 (1996). [DOI] [PubMed] [Google Scholar]
- Romanov R. A., Rogachevskaja O. A., Khokhlov A. A. & Kolesnikov S. S. Voltage dependence of ATP secretion in mammalian taste cells. J Gen Physiol 132, 731 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. D. et al. Glucose-stimulated insulin secretion correlates with changes in mitochondrial and cytosolic Ca2+ in aequorin-expressing INS-1 cells. J Clin Invest 98, 2524 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry F., Boynton R. E., Liu B. & Murphy J. M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res 268, 189 (2001). [DOI] [PubMed] [Google Scholar]
- Palmer G. D. et al. Gene-Induced Chondrogenesis of Primary Mesenchymal Stem Cells in vitro. Molecular Therapy 12, 219 (2005). [DOI] [PubMed] [Google Scholar]
- Alastair M. et al. Chondrogenic Differentiation of Cultured Human Mesenchymal Stem Cells from Marrow. Tissue Engineering 4, 415 (2007). [DOI] [PubMed] [Google Scholar]
- Martin I. et al. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage 9, 112, (2001). [DOI] [PubMed] [Google Scholar]
- Roberts S., Menage J., Sandell L. J., Evans E. H. & Richardson J. B. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee 16, 398 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K. et al. Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. J Clin Invest 121, 640 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A. et al. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Reports 4, 404 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Ye Z., Hu X., Lu L. & Luo Z. Electrical stimulation induces calcium-dependent release of NGF from cultured Schwann cells. Glia 58, 622 (2010). [DOI] [PubMed] [Google Scholar]
- Inoue N. et al. Rapid electrical stimulation of contraction modulates gap junction protein in neonatal rat cultured cardiomyocytes: involvement of mitogen-activated protein kinases and effects of angiotensin II-receptor antagonist. J Am Coll Cardiol 44, 914 (2004). [DOI] [PubMed] [Google Scholar]
- Minina E. et al. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development 128, 4523 (2001). [DOI] [PubMed] [Google Scholar]
- Yoon B. S. & Lyons K. M. Multiple functions of BMPs in chondrogenesis. J Cell Biochem 93, 93 (2004). [DOI] [PubMed] [Google Scholar]
- Kwon H. J., Kurono S., Kaneko Y., Ohmiya Y. & Yasuda K. Analysis of proteins showing differential changes during ATP oscillations in chondrogenesis. Cell Biochem Funct 32, 429 (2014). [DOI] [PubMed] [Google Scholar]
- Martin L Pall. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med 17, 958 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C. J., Kang S. K. & Leung P. C. Adenosine triphosphate-evoked cytosolic calcium oscillations in human granulosa-luteal cells: role of protein kinase C. J Clin Endocrinol Metab 86, 773 (2001). [DOI] [PubMed] [Google Scholar]
- Detimary P., Gilon P. & Henquin J. C. Interplay between cytoplasmic Ca2+ and the ATP/ADP ratio: a feedback control mechanism in mouse pancreatic islets. Biochem J 333, 269 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S. K., Kauri L. M., Qian W. J. & Kennedy R. T. Correlated oscillations in glucose consumption, oxygen consumption, and intracellular free Ca2+ in single islets of Langerhans. J Biol Chem 275, 6642 (2000). [DOI] [PubMed] [Google Scholar]
- Magnus G. & Keizer J. Model of β-cell mitochondrial calcium handling and electrical activity. II. Mitochondrial variables. Am J Physiol 274, C1174 (1998). [DOI] [PubMed] [Google Scholar]
- Varani K. et al. Characterization of adenosine receptors in bovine chondrocytes and fibroblast-like synoviocytes exposed to low frequency low energy pulsed electromagnetic fields. Osteoarthritis Cartilage 16, 292 (2008). [DOI] [PubMed] [Google Scholar]
- De Mattei M. et al. Adenosine analogs and electromagnetic fields inhibit prostaglandin E2 release in bovine synovial fibroblasts. Osteoarthritis Cartilage 17, 252–62 (2009). [DOI] [PubMed] [Google Scholar]
- Vincenzi F. et al. Pulsed electromagnetic fields increased the anti-inflammatory effect of A2A and A3 adenosine receptors in human T/C-28a2 chondrocytes and hFOB 1.19 osteoblasts. PLoS One 8, e65561 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaoning Y., Derya E. A., Pen-hsiu G. C. & Gordana V.-N. Electrical stimulation enhances cell migration and integrative repair in the meniscus. Sci Rep 4, 3674 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt W. S. et al. Intercellular calcium communication regulates platelet aggregation and thrombus growth. J Cell Biol 160, 1151 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S. et al. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci 21, 1975 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahner B. N., Shankar H., Murugappan S., Prasad G. L. & Kunapuli S. P. Nucleotide receptor signaling in platelets. J Thromb Haemost 4, 2317 (2006). [DOI] [PubMed] [Google Scholar]
- Kulyk W. M., Rodgers B. J., Greer K. & Kosher R. A. Promotion of embryonic chick limb cartilage differentiation by transforming growth factor-beta. Dev Biol 135, 424 (1989). [DOI] [PubMed] [Google Scholar]
- Leonard C. M. et al. Role of transforming growth factor-β in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenenous TGFβ and evidence for endogenous TGFβ-like activity. Dev Biol 145, 99 (1991). [DOI] [PubMed] [Google Scholar]
- Duprez D. et al. Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mech Dev 57, 145 (1996). [DOI] [PubMed] [Google Scholar]
- Shen B., Wei A., Tao H., Diwan A. D. & Ma D. D. BMP-2 enhances TGF-beta3-mediated chondrogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in alginate bead culture. Tissue Eng Part A 15, 1311 (2009). [DOI] [PubMed] [Google Scholar]
- Kitaguchi T., Oya M., Wada Y., Tsuboi T. & Miyawaki A. Extracellular calcium influx activates adenylate cyclase 1 and potentiates insulin secretion in MIN6 cells. Biochem J 450, 365 (2013). [DOI] [PubMed] [Google Scholar]
- Roark E. F. & Greer K. Transforming growth factor-β and bone morphogenetic protein-2 act by distinct mechanisms to promote chick limb cartilage differentiation in vitro. Dev Dyn 200, 103 (1994). [DOI] [PubMed] [Google Scholar]
- Costello J. C. et al. Parallel regulation of extracellular ATP and inorganic pyrophosphate: roles of growth factors, transduction modulators, and ANK. Connect Tissue Res 52, 139 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.