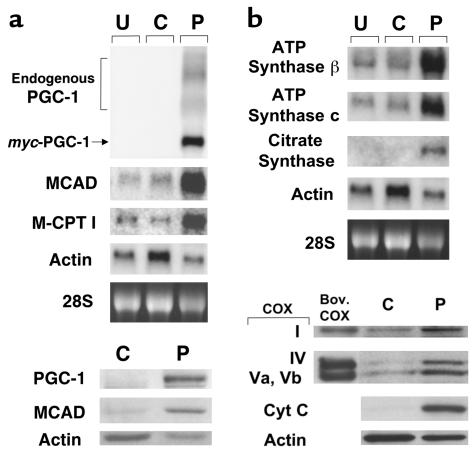
Figure 3.
Forced expression of PGC-1 in cultured rat neonatal cardiac myocytes induces the expression of nuclear and mitochondrial genes involved in multiple mitochondrial energy-transduction/energy-production pathways. (a) The expression of mitochondrial FAO enzyme genes. The autoradiographs represent Northern (top panels) and Western (bottom panels) analyses, respectively. The panels depict the expression of the myc-tagged PGC-1 and endogenous FAO enzyme genes (MCAD and M-CPT I) in rat neonatal cardiac myocytes grown in serum-free culture conditions. Total RNA (15 μg/lane) or protein (10 μg/lane) was isolated from uninfected cells (U), or 48 hours after infection with control adenovirus expressing GFP alone (C), or an adenoviral vector expressing both GFP and PGC-1 (P). As determined by GFP fluorescence, 95–100% of the total cells plated were infected (data not shown). Adenoviral epitope-tagged PGC-1 mRNA is denoted myc-PGC-1 and endogenous PGC-1 transcripts are labeled endogenous PGC-1. The α-actin signal is shown as a control. PGC-1 protein was not detected in extracts from control adenovirus-infected cells by Western blot analysis (bottom panels), even with prolonged exposures. (b) The expression of genes and proteins involved in the TCA cycle, electron transport, and oxidative phosphorylation. The autoradiographs shown at the top represent Northern blot studies performed with total RNA isolated from cardiac myocytes under the conditions described in a. Western blot studies (bottom) were performed with cellular protein extracts prepared from myocytes 4 days after adenoviral infection. Bov. COX, bovine COX protein standards; Cyt C, cytochrome c. All data shown are representative of at least three independent experiments.