With the winter season just around the corner you anticipate numerous patient enquiries and visits related to the use of antibiotics for the common cold and associated morbidities. One day a 45-year-old woman, a recent immigrant from India, presents to your office with sore throat and fever of 2 days' duration. In India her male cousin acquired rheumatic fever following a sore throat for which he did not receive antibiotics. She is now worried about getting rheumatic fever herself and insists on having antibiotics prescribed.
You decide to search the Cochrane Library (www.cochrane.org/reviews/clibintro.htm), to which your practice group subscribes, for evidence of the effectiveness of antibiotics for sore throat to prevent rheumatic fever. You log on and open Issue 3, 2004, and in the search box you enter the terms “(sore throat) and (antibiotics) and (rheumatic fever) and (primary care)” (Fig. 1). You find the review “Antibiotics for sore throat.”1 By printing the review, you obtain 41 pages of text with an abstract including “reviewers' conclusions.” (A lay summary of the review is available at Informed Health Online (www.informedhealthonline.org//item.aspx?tabid=8&review=000023), and that Web site has a direct link to the abstract of the review in the Cochrane Library.) After opening the review, you click on “outline” at the top of the tool bar, and the outline appears on the left of your screen. At the bottom left you click on “metaview graphs” and the outcome “Incidence of acute rheumatic fever within 2 months” is displayed as a forest plot. You notice that the authors have used an odds ratio (0.30, 95% confidence interval [CI] 0.20 to 0.45) as the default statistic for this outcome (Fig. 2). In the forest plot, no weights are assigned to the studies in which no outcomes in both the treatment and control groups occurred. Not being a gambler, you have a poor understanding of odds ratios. You are more familiar with the terms “relative risk,” “absolute risk difference” (“risk difference” as it is called in the Cochrane Library) and “number needed to treat” and you decide to use those statistics instead (Table 1). This is easily done. When you change the statistics in the “methods” box to relative risk, you find similar results as those for odds ratio (because the outcome of rheumatic fever is rare, the odds ratio and relative risk will be numerically close).
Fig. 1: Opening page of the Cochrane Library, Issue 3, 2004. The screen will look like this after you enter your search terms and click on “The Cochrane Database of Systematic Reviews” and then “Complete reviews.” The review “Antibiotics for sore throat” has been ticked. The opening page also gives you a link to the Reviewer's Handbook, which is an extensive document of how Cochrane Reviews are conducted and provides detailed information on statistical methods.
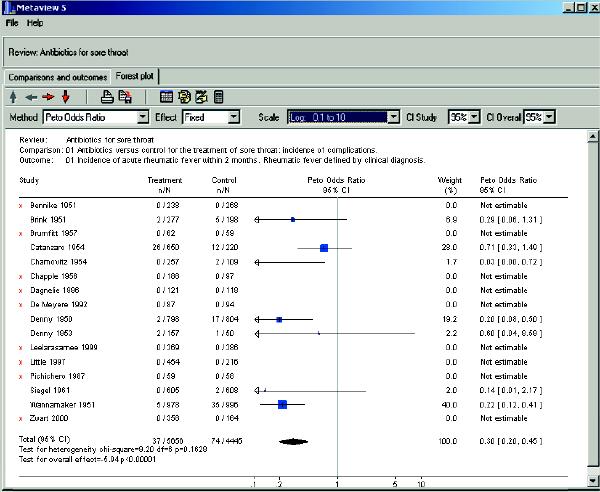
Fig. 2: Forest plot of the outcome “Incidence of acute rheumatic fever within 2 months”1 with odds ratio as the statistic. Results are displayed as odds ratios (see the “Method box” to the left). The vertical line of 1 (unity) under the heading “Peto Odds Ratio” indicates no difference in outcomes between the treatment and control groups. The point estimate of the effect size for each study is illustrated by a box (the size of the box varies according to the sample size of the trial). The 95% confidence interval (CI) is depicted as a horizontal line on each side of the box. If the horizontal line does not cross 1 (unity), the finding of that study is statistically significant. Each study is assigned a weight, but if there are no outcomes in both the treatment and control groups, no weight is assigned. The diamond at the bottom of the display is the typical (summary) odds ratio and includes the 95% CI (0.30, 95% CI 0.20 to 0.45; p < 0.00001). The diamond is located to the left of 1, which indicates a statistically significant reduction in the adverse outcome. The test for heterogeneity is not significant (p = 0.1628).
Table 1
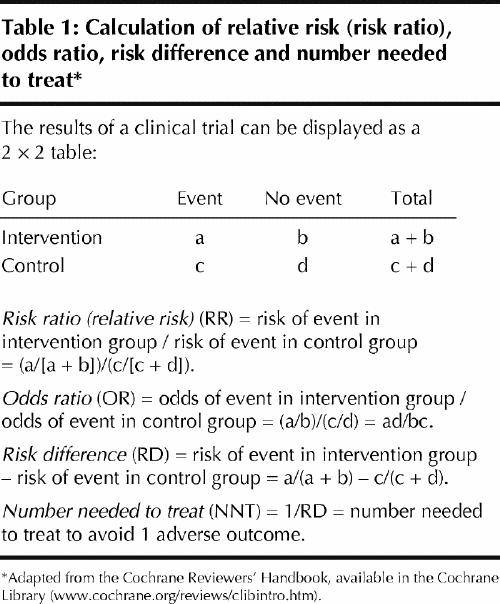
In the methods section of the review you note that the reviewers made extensive efforts to identify eligible studies, and it would seem inappropriate to discard important information because there were no outcomes in both the treatment and control groups (indicating that there may be no advantage of the intervention under study). When you change the statistics to risk difference (–0.01, 95% CI –0.02 to –0.01), all of the studies are weighted (Fig. 3). A risk difference of 1% between the treatment and the control group of having the adverse outcome may convey a different message than an odds ratio of 0.30 would. By taking the inverse of the risk difference (in this case 1/0.01) you obtain the number needed to treat (100, 95% CI 50 to 100). It tells you that, on average, you need to treat 100 patients with sore throat with antibiotics to avoid 1 case of rheumatic fever. You note that the test for heterogeneity is statistically significant (p < 0.00001), which indicates that the effect size varies between studies, making the results less robust. In addition, you note that, in the studies conducted in the 1990s, there were no cases of rheumatic fever among 2484 patients enrolled. The reviewers performed a secondary analysis in which they separated studies published before and after 1975 and found no cases of rheumatic fever after 1975. As expected, there is no heterogeneity among the studies published after 1975, as there were no adverse outcomes in any group.
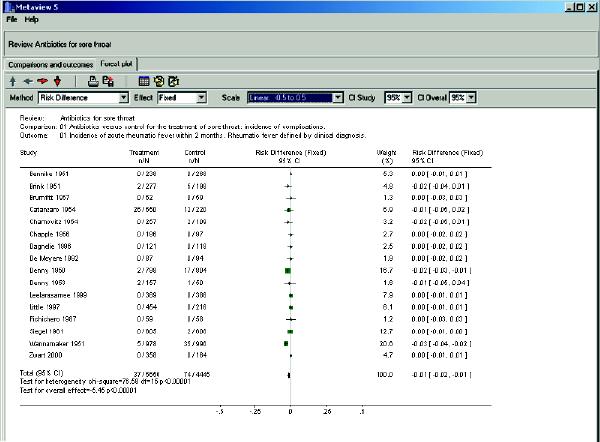
Fig. 3: Forest plot of the outcome “Incidence of acute rheumatic fever within 2 months”1 with risk difference as the statistic. Same comparison and outcome as in Fig. 2, but in the “Method box” the statistical test has been changed to “risk difference,” and the scale has been changed to “–0.5 to 0.5” to make it easier to visualize the results of individual studies. The vertical line under the heading “Risk difference (fixed)” represents no difference in outcomes between the treatment and control groups (unity is now 0). All studies have now been assigned a weight. The risk difference is –0.01 (95% CI –0.02 to –0.01), p < 0.0001. The test for heterogeneity is statistically significant (p < 0.0001), which indicates that there is study heterogeneity with regard to the effect size.
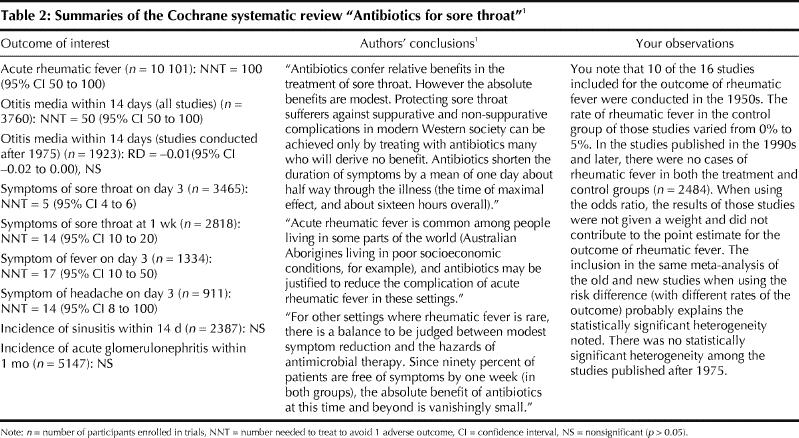
You summarize your findings in Table 2, and you have good evidence not to prescribe antibiotics for sore throat to prevent rheumatic fever in your patient. There may be subpopulations in Canada in whom the base rate of rheumatic fever is very high, as is the case in Australian Aborigines living in poor socioeconomic conditions, and therefore the use of antibiotics for sore throat is justified.
Table 2
Arne Ohlsson Kathie Clark
β See related article page 747
Supplementary Material
Footnotes
Arne Ohlsson is Professor in the Departments of Paediatrics, Obstetrics and Gynaecology, and Health Policy Management and Evaluation, University of Toronto, Toronto, Ont., and the Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, Ont. He is Director of the Canadian Cochrane Network and Centre. Kathie Clark is Co-director of the Canadian Cochrane Network and Centre, Faculty of Health Sciences, McMaster University, Hamilton, Ont.
For a trial use of the Cochrane Library, please refer to www.cmaj.ca for details.
Reference
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


