FIG. 8.
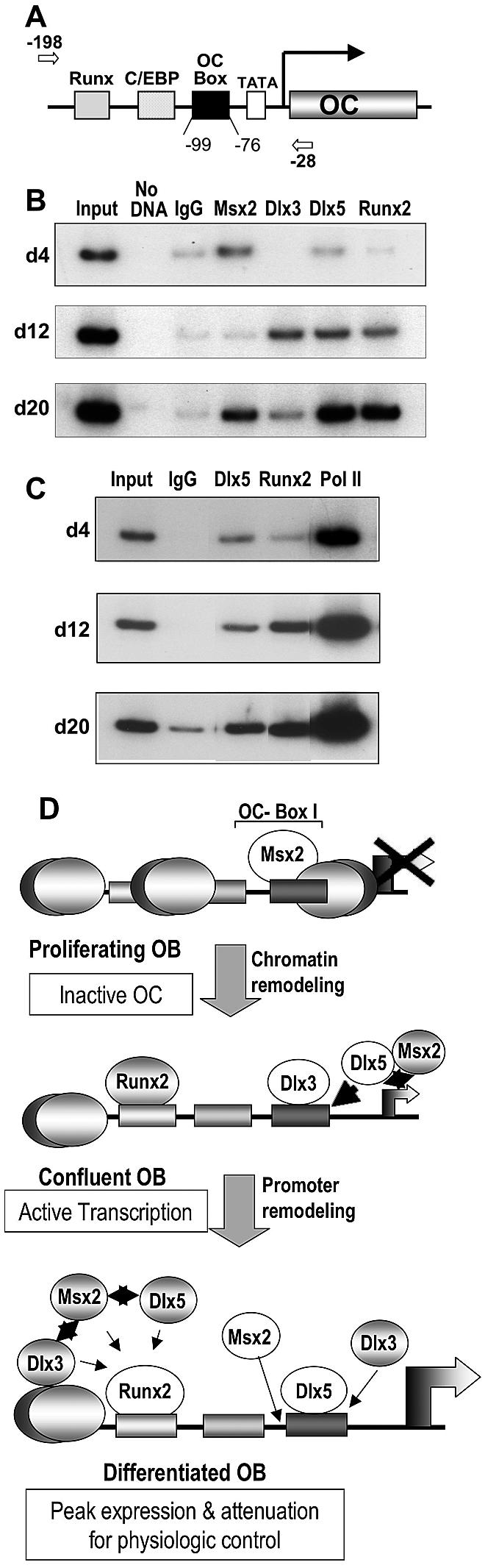
In vivo occupancy of HD proteins in the proximal OC promoter during osteoblast growth and differentiation. (A) The primers used to amplify the key regulatory elements present in the proximal OC promoter fragment in the ChIP assays are indicated in the top line. The arrows indicate the position of the forward (−190) and reverse (−28) primers. (B) Nuclei were prepared from rat calvaria-derived osteoblast cultures at the indicated stages of ROB differentiation (days 4, 12, and 20) for ChIP assays. Formaldehyde-cross-linked chromatin samples were used directly for immunoprecipitation reactions with 2 μg of each Msx (1 and 2), Dlx3, Dlx5 (Y20), PolII, and Runx2 antibody. Immunocomplexes were reversed at 68°C overnight, and the DNA fragments were purified and assayed by PCR. Normal IgG (2 μg) was used as a control for each time point. Input represents 0.2% of each chromatin fraction used for immunoprecipitation. The ChIP data presented are representative of four experiments in which all threetime points were derived from the same osteoblast preparation. (C) Nuclei were derived from calvarial osteoblasts as for panel B for ChIP assays, using the indicated antibodies to demonstrate increased transcription of OC with increased occupancy of Dlx5 and Runx2. (D) Schematic illustration of the ChIP results showing molecular switching in HD protein association with OC chromatin for negative and positive regulation of transcription. The proximal promoter regulatory elements during osteoblast growth and differentiation of the rat OC gene are represented with bound transcription factors at each stage of osteoblast differentiation. The stages are described on the left with the OC transcriptional status indicated. In proliferating cells where OC is not expressed and lacks DNase I hypersensitivity, Msx2 may be bound to linker DNA (between nucleosomes) or to nucleosomal DNA associated with histone deacetylases. Options for occupancy of the HD site by Dlx3 and Dlx5, as well as the potential for protein-protein interactions of Runx2 with Msx2, Dlx3, and Dlx5 to attenuate OC transcription, are shown. Msx2-Dlx5/Dlx3 heterodimerization may also contribute to physiological levels of OC transcription (see text for details). White circles indicate prominent occupancy of the factor at the indicated stages.
