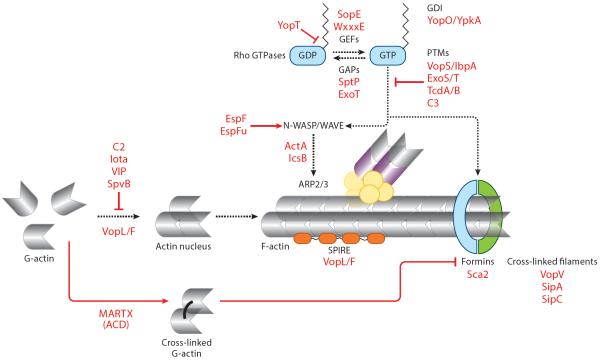
Figure 1.
Regulation of actin dynamics by bacterial toxins and effector proteins. Bacterial pathogens have evolved multiple strategies to both positively and negatively regulate actin dynamics. Actin polymerization is inhibited through ADP-ribosylation of G-actin into nonpolymerizable monomers by the C2 toxin, the iota toxin, the VIP (vegetative insecticidal protein) toxin, and the Salmonella effector SpvB. ACD (actin cross-linking domain) toxin–mediated actin cross-linking poisons the formin family of actin nucleators. Actin polymerization is inhibited by bacterial effectors through the removal of membrane-bound Rho GTPases via either sequestration of the lipid group by YopO/YpkA or proteolytic cleavage of the lipid group from Rho GTPases by YopT. Additionally, inactivation of Rho GTPases via effector GAP (GTPase-activating protein) activity of SptP and ExoT shuts off actin polymerization. Rho GTPases undergo posttranslational modification (PTM) by ADP-ribosylation via C3 toxins and effectors ExoS and ExoT and by AMPylation by VopS and IbpA, preventing Rho GTPase activation of actin polymerization. Bacterial pathogens have also evolved mechanisms to induce actin dynamics through various mechanisms. VopL/F, Sca2, and SipC directly nucleate actin. Additionally, VopV, SipC, and SipA stabilize actin filaments by cross-linking. Bacterial effectors directly activate or function as nucleation-promoting factors to promote Arp2/3-mediated actin nucleation. Activation of Rho GTPase signaling by SopE- and WxxxE-family members of bacterial GEFs (guanine nucleotide exchange factors) also induces actin polymerization.