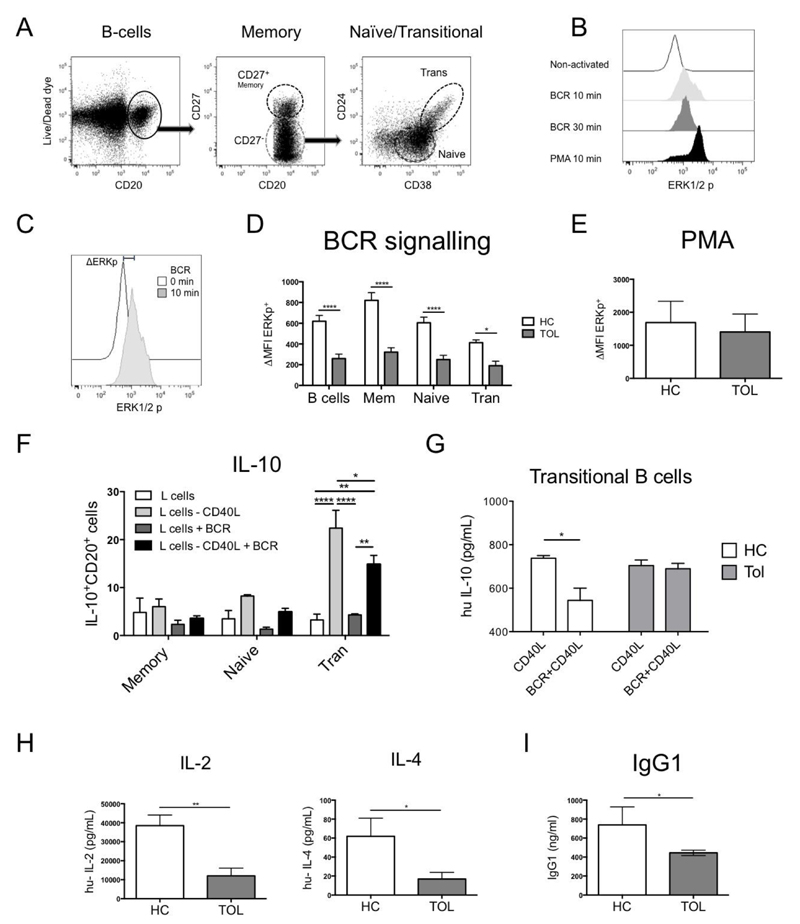
Fig. 2. B-cells from tolerant recipients display a reduced ERK signalling after BCR activation.
A) A phospho-flow B-cell panel was designed to identify p-ERK in B-cell subsets within PBMCs. B) A time course of p-ERK was measured in PBMCs after BCR-activation [anti-IgM (20μg/ml)/anti-IgG (20μg/ml)] during 0, 10 and 30min at 37°C, using PMA (0.1μM) for 10min as a positive control. C) Δp-ERK was defined as the difference between the p-ERK MFI from a non-activated and a 10min BCR-activated sample. D) The BCR signalling was measured in 1x106 PBMCs from healthy control (HC=9) and tolerant (Tol=9) patients after BCR-activation or after E) PMA (0.1μM) for 10min. F) IL-10 production after BCR/CD40 activation was measured by adding 0.5x105 non-transfected or CD40L-transfected L-cells in 0.5x106 non-activated or BCR-activated B-cell subsets from healthy leukocytes retained in filtering cones after 3 days of culture by intracellular staining. G) Levels of IL-10 were measured in the supernatants of 10x104 sorted transitional B-cells from 3 HC and 3 Tol recipients activated with 1x103 non-transfected or CD40L-transfected L-cells, with or without BCR activation for 3 days. H) IL-2 and IL-4 production from isolated CD4+T-cells of HC=6 and Tol=7 were measured after 48h of activation with CD3/CD28 beads (1:2 ratio). Detection limit for IL-2 and IL-4 were 2.6 and 4.9pg/ml, respectively. I) Levels of IgG1 were measured in the sera from HC and Tol patients. Mann-Whitney test and two-way RM ANOVA test with a Sidak’s multiple comparisons test were used, **** P<0.0001, *** P<0.001, ** P<0.01 and * P<0.05 were considered significant.