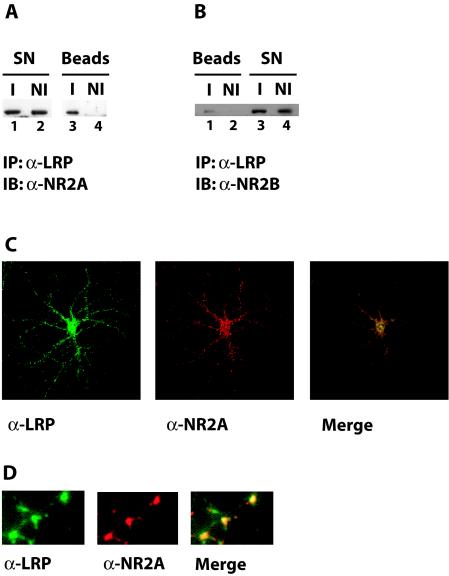
FIG. 5.
(A) Coimmunoprecipitation of LRP1 and NR2A. Whole-cell lysates were prepared from cultured primary rat embryonic cortical neurons and used for immunoprecipitation with a polyclonal anti-LRP1 antibody (I) or with nonimmune control serum (NI). Immune complexes were recovered on protein A agarose beads and analyzed by SDS-polyacrylamide gel electrophoresis. Western blotting with an antibody against the NMDA receptor subunit NR2A revealed coprecipitation of NR2A with LRP1 (lane 3). No NR2A was detected in the nonimmune control sample (lane 4). IB, immunoblotting; SN, supernatant. (B) Coimmunoprecipitation of LRP1 and NR2B. The experiment was conducted as described in the legend to Fig. 5A, except that Western blotting was performed with an antibody against the NMDA receptor subunit NR2B. Lane 1 shows coimmunoprecipitation of NR2B with LRP1. (C) Partial colocalization of LRP1 and NR2A. Rat hippocampal neurons in glial cell coculture were examined by indirect immunofluorescence. Costaining was performed with an affinity-purified LRP1 antibody and an antibody against the NMDA receptor subunit NR2A. Bound α-LRP1 was visualized with an Alexa 488-labeled secondary antibody (green fluorescence), and bound α-NR2A was visualized with an Alexa 594 conjugate (red fluorescence). Photographic overlay of images acquired by confocal laser scanning microscopy revealed partial colocalization of LRP1 and NR2A (yellow). (D) Higher magnification of a dendritic process from rat embryonic neurons stained as described for panel C.