Abstract
Background
The association between institutional volume and outcomes has been demonstrated for cardiac catheterization among adults, but less is known about this relationship for patients with congenital heart disease (CHD) undergoing cardiac catheterization.
Methods
Within the IMPACT® (Improving Pediatric and Adult Congenital Treatment) Registry, we identified all catheterizations between January 2011 and March 2015. Hierarchical logistic regression, adjusted for patient and procedural characteristics, was used to determine the association between annual catheterization lab volume and occurrence of a major adverse event (MAE).
Results
Of 56,453 catheterizations at 77 hospitals, an MAE occurred in 1,014 (1.8%) of cases. In unadjusted analysis, an MAE occurred in 2.8% (123/4,460) of cases at low-volume hospitals (<150 procedures annually), as compared with 1.5% (198/12,787), 2.0% (431/21,391), and 1.5% (262/17,815) of cases at medium- (150-299 annual procedures), high- (300-499 annual procedures), and very high-volume (≥500 procedures annually) hospitals, respectively, p<0.001. After multivariable adjustment, this significant relationship between annual procedure volume and occurrence of an MAE persisted. Compared to low-volume programs, the odds of an MAE was 0.55 (95% confidence interval [CI] 0.35, 0.86, p=0.008), 0.62 (95% CI 0.41, 0.95, p=0.03), and 0.52 (95% CI 0.31, 0.90, p=0.02) at medium-, high-, and very high-volume programs, respectively.
Conclusions
Although the risk of MAE after cardiac catheterization in patients with CHD is low at all hospitals, it is higher among hospitals with fewer than 150 cases annually. These results support the notion that centers meeting this threshold volume for congenital cardiac catheterizations may achieve improved patient outcomes.
Keywords: congenital heart disease, cardiac catheterization
The relationship between institutional procedural volume and clinical outcomes has been described for several cardiovascular procedures, including coronary revascularization procedures and placement of implantable cardioverter defibrillators (ICDs)(1,2). This “volume-outcome” relationship extends to the field of pediatric cardiovascular surgery, with higher peri-procedural mortality rates at low-volume programs(3-10).
Less is known regarding the relationship between institutional procedural volume and occurrence of adverse events among pediatric and adult patients with congenital heart disease (CHD) undergoing cardiac catheterization. Prior work has suggested an association between operator-level experience and incidence of adverse events, suggesting that individual interventionalists undergo a “learning curve”(11). Moreover, a recent study evaluating the relationship between institutional volume and catastrophic adverse events in children and young adults undergoing cardiac catheterization found a reduced risk of catastrophic events at higher volume centers(12). However, this study was performed using administrative data, in which limited clinical detail makes adjustment for patient- and procedural-level variables challenging. Therefore, the relationship between institutional volume and outcomes for cardiac catheterization in pediatric and adult patients with CHD is less well-defined.
The NCDR's (National Cardiovascular Data Registry) IMPACT® (IMproving Pediatric and Adult Congenital Treatment) Registry is the largest clinical registry, to date, of percutaneous CHD procedures. The registry collects information on pediatric patients with congenital or acquired heart disease and adult patients with CHD undergoing diagnostic and interventional cardiac catheterization procedures(13). As a result, IMPACT has both the size and clinical detail to evaluate the relationship between institutional procedural volume and the occurrence of adverse events with appropriate adjustment for patient- and procedure-level factors. The goal of this project was to examine whether hospitals performing more cardiac catheterizations on pediatric or adult patients with CHD experience fewer major adverse events as compared with hospitals performing lower volumes of similar procedures.
Methods
Study Population
IMPACT is a U.S.-based registry collecting information on pediatric and adult patients with CHD undergoing diagnostic or interventional cardiac catheterization. Details regarding registry development and its basic design have been previously published(13). In brief, centers performing cardiac catheterization on pediatric patients with congenital or acquired heart disease and adult patients with CHD are eligible for voluntary enrollment in IMPACT. Participating centers collect detailed information on all consecutive patients undergoing a catheterization procedure, including information regarding patient demographics, clinical data, and detailed procedural information. IMPACT uses standardized data elements and definitions and is subject to rigorous quality assurance standards. The current study used data from IMPACT v1.0.1. A comprehensive description of the IMPACT Registry data elements and definitions is available at: https://www.ncdr.com/WebNCDR/impact/home/datacollection.
From January 2011 through March 2015, a total of 57,032 diagnostic or interventional cardiac catheterization procedures at 77 US centers were identified. Procedures that could not be assigned to a procedural risk group (n=14)(14), had missing information regarding single ventricle status (n=217), or had missing data on adverse events (n=348) were excluded. The final study cohort comprised 56,453 cardiac catheterization procedures (52,295 episodes of care) at 77 centers.
Study Outcome
The primary outcome was the occurrence of a major adverse event, defined as occurrence of any of the following: cardiac arrest; arrhythmia requiring permanent pacemaker; cardiac tamponade (requiring pericardial drainage); air embolus; embolic stroke within 72 hours of the cardiac catheterization; new requirement for dialysis, extracorporeal membrane oxygenation (ECMO), or left ventricular assist device (LVAD); unplanned cardiac, vascular, or other surgery (due to catheterization complication); or subsequent cardiac catheterization (due to catheterization complication). Unless otherwise specified, adverse events are coded up to 30 days following the catheterization procedure, aside from unplanned surgery and subsequent cardiac catheterization which are coded until the time of hospital discharge. Death was excluded from the primary outcome because death during the hospitalization cannot definitively be attributed to cardiac catheterization and could have resulted from other in-hospital events (e.g. cardiac surgery)(15). As a secondary outcome, however, death was added to the primary outcome described above.
Categorization of Procedure Volume
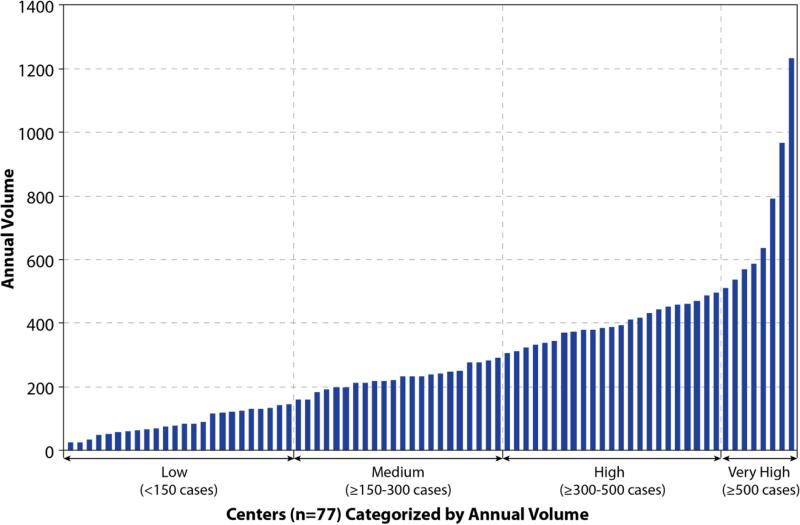
Annual procedure volume was calculated based upon the number of cases submitted during the study period divided by the number of months a center submitted data to IMPACT multiplied by 12. This was chosen over individual annual catheterization volume because it was felt that the experience of an individual center was durable and unlikely to be prone to year-to-year variation. Centers were categorized into four groups based upon annual procedural volume: low-volume (<150 cases per year), medium-volume (150-299 cases per year), high-volume (300-499 cases per year), and very high-volume (≥500 cases per year). These cut-points were defined a priori, and roughly correlated with quartiles of hospitals’ volumes (Figure 1).
Figure 1.
Annual Center Volume. Annual case volume for each of the 77 participating centers.
Patient and Procedural Risk Factors
We adjusted for a number of pre-defined patient and procedural risk factors to account for center differences in case-mix. These included age at the time of cardiac catheterization (categorized as neonates [<30 days], infants [≥30 days to ≤1 year], children [1≤18 years], and adults [>18 years of age]) and procedure status (elective, urgent, emergent, or salvage). We also adjusted for pre-procedure sepsis and patients’ requirement for inotrope therapy, ECMO, or LVAD prior to the procedure. Additionally, we adjusted for medical co-morbidities (chronic lung disease and renal insufficiency), single ventricle physiology, and the presence of a genetic or congenital condition (i.e. 22q11 deletion, Alagille syndrome, congenital diaphragmatic hernia, Trisomy-21, heterotaxy syndrome, Marfan syndrome, Noonan syndrome, Rubella, Trisomy-13, Trisomy-18, Turner syndrome, Williams-Beuren syndrome).
We also sought to adjust for severity of illness in our model, using the concept of hemodynamic vulnerability. Hemodynamic vulnerability was determined using previously published data from the Congenital Cardiac Catheterization Project on Outcomes (C3PO), a collaboration of eight centers collecting data on catheterization procedures performed for congenital heart disease(16). In brief, four hemodynamic variables (systemic ventricular end diastolic pressure, systemic arterial saturation, mixed venous saturation, and main pulmonary artery pressure) are known to be independently associated with experiencing a high-severity adverse event following cardiac catheterization and are used to classify a patient as hemodynamically vulnerable. Thresholds for each variable differ based upon whether a patient has single or 2-ventricle physiology and specifically are: 1) systemic arterial saturation <95% in 2-ventricle and <78% in single-ventricle patients; 2) mixed venous saturation <60% in 2-ventricle and <50% in single-ventricle patients; 3) main pulmonary artery systolic pressure ≥45mmHg in 2-ventricle patients and a main pulmonary artery mean pressure ≥17mmHg in single-ventricle patients; and 4) a systemic ventricular end diastolic pressure ≥18mmHg (regardless of underlying anatomy). For each variable, patients were classified as “yes” if their catheterization procedure met criteria for hemodynamic vulnerability, “no” if their catheterization data did not meet criteria for hemodynamic vulnerability, or “missing” if data were missing on the relevant hemodynamic parameter. This third category of “missing” was used given that the reasons for missing data were not known and exclusion of records with missing hemodynamic data had the potential to result in model bias. For example, it was unknown whether patients missing hemodynamic data were patients for whom the particular hemodynamic variable was not relevant or whether they were critically ill patients whose clinical status precluded a thorough hemodynamic assessment. In total, there were 5,285 (9.4%) records missing data on systemic arterial saturation, 7,500 (13.3%) records with missing data on mixed venous saturation, 20,728 (36.7%) records missing data on systemic ventricle end diastolic pressure, and 15,268 (27.0%) records with missing data on main pulmonary artery pressures.
We also adjusted for procedural risk factors, including procedure-type risk group. To do this we utilized procedure risk groups previously derived from C3PO data (14) (Table 1). Briefly, congenital cardiac catheterization encompasses a wide variety of interventional procedure types, each associated with different degrees of risk. Given the broad range of procedure types, adjustment for each individual procedure is not feasible. Procedure-type risk categories were developed to overcome this issue and to establish a classification system whereby procedures of similar risk are grouped. Within C3PO, four categories of procedural risk were created and validated (Category 1= procedures associated with lowest risk vs. Category 4= procedures associated with highest risk). The risk groups were derived using a combination of empirically derived data and expert consensus and were found to have good discrimination between each of the categories. For catheterization lab visits where more than one procedure was performed, the case was categorized according to the procedure of highest risk.
Table 1.
Procedure-Type Risk Categories
| Risk Category 1 | Risk Category 2 | Risk Category 3 | Risk Category 4 | |
|---|---|---|---|---|
| Diagnostic Case | Age ≥1 year | Age ≥1 month <1 year | Age <1 month | |
| Valvuloplasty | Pulmonary valve ≥1 month | Aortic valve ≥1 month Pulmonary valve <1 month Tricuspid valve |
Aortic valve <1 month Mitral valve |
|
| Device or coil closure | Venous Collateral LSVC |
ASD/PFO PDA Fontan fenestration Systemic to pulmonary artery collaterals |
Systemic surgical shunt Baffle leak Coronary fistula |
VSD Perivalvar leak |
| Balloon angioplasty | RVOT Aorta dilation <8atm |
Pulmonary artery < 4 vessels Pulmonary artery ≥ 4 vessels all <8atm Aorta >8atm or CB Systemic artery (not aorta) Systemic surgical shunt Systemic to pulmonary collaterals Systemic vein |
Pulmonary artery ≥4 vessels Pulmonary vein |
|
| Stent placement | Systemic vein | RVOT Aorta Systemic artery (not aorta) |
Ventricular septum Pulmonary artery Pulmonary vein Systemic surgical shunt Systemic pulmonary collateral |
|
| Stent re-dilation | RVOT Atrial septum Aorta Systemic artery (not aorta) Systemic vein |
Pulmonary artery Pulmonary vein |
Ventricular Septum | |
| Other | Myocardial biopsy | Snare foreign body Transseptal puncture |
Atrial septostomy Recanalization of jailed vessel in stent Recanalization of occluded vessel |
Atrial septum dilation and stent Any catheterization <4 days after surgery Atretic valve perforation |
Abbreviations: LSVC, left superior vena cava; ASD, atrial septal defect; PFO, patent foramen ovale; PDA, patent ductus arteriosus; RVOT, right ventricular outflow tract; atm, atmospheres; CB, cutting balloon; VSD, ventricular septal defect
Data source: Bergersen L, Gauvreau K, Marshall A, et al. Circ Cardiovasc Inter. 2011; 4: 188-194.
Statistical Analysis
Baseline patient characteristics for low-, medium-, high-, and very high-volume programs were compared using one-way analysis of variance for continuous variables and chi-square or Fisher's exact test for categorical variables. Within the study population, there were a small number of patients missing data on one of the relevant covariates (excluding those with missing hemodynamic data as detailed above). In total, 3.4% of patients were missing data for at least one covariate. Only 0.38% of patients were missing data for more than one covariate. Data were imputed using IVEware software (University of Michigan, Ann Arbor, MI), aside from the missing hemodynamic data which were handled as described above.
A multivariable hierarchical logistic regression model that adjusted for patient and procedural characteristics (as described above) was used to evaluate the association between annual hospital volume and occurrence of a major adverse event. Covariates (listed above) were selected based on clinical relevance and previously published literature. All covariates were included in the final model, regardless of statistical significance. A hierarchical model was used to account for nesting of patients within hospitals. In these multivariable analyses, hospitals were modeled as random effects whereas patient and procedural factors were modeled as fixed effects. Moreover, to identify whether or not the association between institutional volume and occurrence of adverse events differed based upon procedural risk or based upon age, a hospital volume-by-procedure-risk-group interaction and a hospital volume-by-age interaction were examined. The primary model treated hospital volume as a categorical variable. A second analysis was performed treating hospital volumes as a continuous variable, after first testing for a non-linear association using restricted cubic splines. If no non-linear association was detected, volume was treated as a linear continuous variable. A similar analytic approach was used for the secondary outcome, wherein death was included as part of the composite end point. All analyses were evaluated using 2-sided tests of significance with a threshold of p<0.05. Analyses were performed with SAS 9.3 (SAS Institute, Cary, NC) or R version 2.11.1(17). The study was conducted on de-identified quality improvement registry data and did not meet criteria for requirement of informed consent. The IMPACT Registry's Research and Publications Committee approved the final manuscript draft. Dr. Jayaram was supported by a T32 training grant (HL110837) from the National Heart Lung and Blood Institute. Dr. O'Byrne received support from the NIH [T32 HL007915] and Entelligence Young Investigator grant. Dr. Chan is supported by an R01 Award (1R01HL123980) from the National Heart Lung and Blood Institute. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
The mean annual case volume per hospital was 286± 214, and the median annual case volume was 240 (inter-quartile range: 130 to 387; total range: 24 to 1233) (Figure 1). Among the 77 hospitals, 24 (31.2% of hospitals and 7.9% of cases) were low-volume centers, 22 (28.6% of hospitals and 22.7% of cases) medium-sized centers, 23 (29.9% of hospitals and 37.9% of cases) high-volume centers, and 8 (10.4% of hospitals and 31.6% of cases) very high-volume centers.
Of the 56,453 cardiac catheterization cases, the majority were performed in patients under 18 years of age, with 3,457 (6.1%) in neonates, 11,108 (19.7%) in infants, and 33,464 (59.3%) in children, whereas 8,424 (14.9%) were performed in adults. Patient and procedural characteristic by the four hospital categories of procedural volume are summarized in Tables 2 and 3. Low-volume-programs had a higher proportion of adult patients compared to larger-sized programs. Low-volume programs also had a smaller percentage of single ventricle patients. Genetic conditions were slightly more prevalent at very-high volume programs. Low-volume programs had a smaller percentage of their patients undergoing procedures classified within the highest procedural risk group. Very-high volume programs were more likely to have cases scheduled as urgent procedures and less likely to have procedures scheduled electively.
Table 2.
Baseline patient characteristics of study cohort stratified by annual hospital volume
| Hospital Annual Volume | p-value | ||||
|---|---|---|---|---|---|
| <150 cases/year n=4,460 | ≥150-300 cases/year n=12,787 | ≥300-500 cases/year n=21,391 | ≥500 cases/year n=17,815 | ||
| Age--no. (%) | < 0.001 | ||||
| <30 days | 215 (4.8) | 824 (6.4) | 1,363 (6.4) | 1,055 (5.9) | |
| ≥30 days to ≤1 year | 785 (17.6) | 2,650 (20.7) | 4,238 (19.8) | 3,435 (19.3) | |
| >1 year to ≤ 18 years | 1,998 (44.8) | 7,344 (57.4) | 12,716 (59.4) | 11,406 (64.0) | |
| >18 years | 1,462 (32.8) | 1,969 (15.4) | 3,074 (14.4) | 1,919 (10.8) | |
| Female Sex--no. (%) | 2,163 (48.5) | 6,040 (47.2) | 9,989 (46.7) | 8,164 (45.8) | 0.005 |
| Weight-- mean ± s.d.* | 40.0 ± 34.8 | 30.4 ± 29.3 | 29.5 ± 28.3 | 28.4 ± 26.4 | < 0.001 |
| Single Ventricle--no. (%) | 554 (12.4) | 2,568 (20.1) | 4,806 (22.5) | 3,272 (18.4) | < 0.001 |
| Genetic Condition--no. (%)† | 441 (9.9) | 1,351 (10.6) | 2,271 (10.6) | 2,135 (12.1) | < 0.001 |
| Medical co-morbidities--no. (%)‡ | 1,392 (31.3) | 3,999 (31.3) | 7,248 (33.9) | 5,539 (31.1) | < 0.001 |
| Sepsis--no. (%)§ | 20 (0.5) | 56 (0.4) | 157 (0.7) | 88 (0.5) | < 0.001 |
| Inotrope Need--no. (%)∥ | < 0.001 | ||||
| No | 4,252 (96.0) | 11,973 (94.1) | 19,503 (91.8) | 16,181 (91.3) | |
| On before case, on or off at end | 113 (2.6) | 578 (4.5) | 1,096 (5.2) | 963 (5.4) | |
| Started during case or used for measurement only | 62 (1.4) | 166 (1.3) | 636 (3.0) | 573 (3.2) | |
| ECMO Use--no. (%)¶ | 0.007 | ||||
| In place at start of procedure | 21 (0.5) | 117 (0.9) | 211 (1.0) | 179 (1.0) | |
| Not used or electively initiated during procedure | 4,436 (99.5) | 12,613 (99.1) | 21,122 (99.0) | 17,608 (99.0) | |
| LVAD Use--no. (%)# | 0.62 | ||||
| In place at start of procedure | 3 (0.1) | 19(0.1) | 30 (0.1) | 25 (0.1) | |
| Not used or electively initiated during procedure | 4,450 (99.9) | 12,708 (99.9) | 21,288 (99.9) | 17,756 (99.9) | |
| Cardiac Index-- mean ± s.d.** | 3.8 ± 1.4 | 3.8 ± 1.3 | 3.8 ± 1.4 | 3.9 ± 1.3 | < 0.001 |
| Single Ventricle Hemodynamic Data | |||||
| Systemic Saturation-- mean± s.d.†† | 84.1 ± 9.2 | 83.9 ± 9.8 | 84.0 ± 10.6 | 83.6 ± 10.1 | 0.27 |
| Mixed Venous Saturation-- mean± s.d.‡‡ | 62.2 ± 11.4 | 62.0 ± 11.2 | 61.9 ± 11.3 | 61.3 ± 11.4 | 0.08 |
| MPA Mean Pressure -- mean± s.d.§§ | 15.9 ± 7.3 | 14.9 ± 6.8 | 16.1 ± 7.3 | 16.0 ± 6.9 | < 0.001 |
| Non-Single Ventricle Hemodynamic Data | |||||
| Systemic Saturation-- mean± s.d.∥∥ | 94.6 ± 6.9 | 94.6 ± 6.9 | 94.4 ± 7.2 | 94.8 ± 7.1 | < 0.001 |
| Mixed Venous Saturation-- mean± s.d.¶¶ | 70.5 ± 9.8 | 70.4 ± 9.3 | 70.2 ± 9.6 | 69.8 ± 9.5 | < 0.001 |
| MPA Systolic Pressure -- mean± s.d.## | 32.1 ± 16.6 | 31.6 ± 16.4 | 31.1 ± 16.2 | 31.7 ± 16.8 | 0.001 |
| Systemic Ventricular EDP ≥ 18mmHg | < 0.001 | ||||
| Yes-- no. (%) | 228 (5.1) | 514 (4.0) | 949 (4.4) | 993 (5.6) | |
| No-- no. (%) | 2,676 (60.0) | 7,353 (57.5) | 12,615 (59.0) | 10,397 (58.4) | |
| Missing-- no. (%) | 1,556 (34.9) | 4,920 (38.5) | 7,827 (36.6) | 6,425 (36.1) | |
| Saturation <95% (non-SV) or <78% (SV) | < 0.001 | ||||
| Yes-- no. (%) | 1,137 (25.5) | 3,150 (24.6) | 5,655 (26.4) | 4,402 (24.7) | |
| No-- no. (%) | 2,773 (62.2) | 8,217 (64.3) | 14,096 (65.9) | 11,738 (65.9) | |
| Missing-- no. (%) | 550 (12.3) | 1,420 (11.1) | 1,640 (7.7) | 1,675 (9.4) | |
| MV Saturation <60% (non-SV) or <50% (SV) | < 0.001 | ||||
| Yes-- no. (%) | 452 (10.1) | 1,337 (10.5) | 2,397 (11.2) | 2,101 (11.8) | |
| No-- no. (%) | 3,298 (73.9) | 9,275 (72.5) | 16,243 (75.9) | 13,850 (77.7) | |
| Missing-- no. (%) | 710 (15.9) | 2,175 (17.0) | 2,751 (12.9) | 1864 (10.5) | |
| PA Systolic Pressure ≥45 (non-SV) or Mean | |||||
| Pressure ≥17mmHg (SV) | < 0.001 | ||||
| Yes-- no. (%) | 645 (14.5) | 1,756 (13.7) | 3,190 (14.9) | 2,742 (15.4) | |
| No-- no. (%) | 2,836 (63.6) | 7,753 (60.6) | 13,775 (64.4) | 12,384 (69.5) | |
| Missing-- no. (%) | 979 (22.0) | 3,278 (25.6) | 4,426 (20.7) | 2,689 (15.1) | |
Abbreviations: no., number; s.d., standard deviation; ECMO, extracorporeal membrane oxygenation; LVAD, left ventricular assist device; MPA, main pulmonary artery; EDP, end diastolic pressure; MV, mixed venous; SV, single ventricle; PA, pulmonary artery
239 patients (22 from small, 62 from medium, 115 from large, 40 from very large volume programs) with missing information for weight
255 patients (18 from small, 35 from medium, 53 from large, 149 from very large volume programs) with missing information for genetic condition
90 patients (12 from small, 25 from medium, 26 from large, 27 from very large volume programs) with missing information for medical co-morbidities
899 patients (60 from small, 186 from medium, 389 from large, 264 from very large volume programs) with missing information for sepsis
357 patients (33 from small, 70 from medium, 156 from large, 98 from very large volume programs) with missing information for inotrope need
146 patients (3 from small, 57 from medium,58 from large, 28 from very large volume programs) with missing information for ECMO use
174 patients (7 from small, 60 from medium, 73 from large, 34 from very large volume programs) with missing information for LVAD use
11, 093 patients (1,098 from small, 3,270 from medium,4,033 from large, 2,692 from very large volume programs) with missing information for cardiac index
840patients (34 from small, 203 from medium, 326 from large, 277 from very large volume programs) with missing information for SV systemic saturation
1,372 patients (66 from small, 337 from medium, 604 from large, 365 from very large volume programs) with missing information for SV MV saturation
2,251 patients (123 from small, 608 from medium, 904 from large,616 from very large volume programs) with missing information for SV MPA Mean Pressure
4,445 patients (516 from small, 1,217 from medium, 1,314 from large, 1,398 from very large volume programs) with missing information for non-SV systemic saturation
6,128 patients (644 from small, 1,838 from medium, 2,147 from large, 1,499 from very large volume programs) with missing information for non-SV MV saturation
9,121 patients (856 from small, 2,670 from medium, 3,522 from large, 2,073 from very large volume programs) with missing information for non-SV MPA Systolic Pressure
Table 3.
Baseline procedural characteristics of study cohort stratified by annual hospital volume
| Hospital Annual Volume | p-value | ||||
|---|---|---|---|---|---|
| <150 cases/year n=4,460 | ≥150-300 cases/year n=12,787 | ≥300-500 cases/year n=21,391 | ≥500 cases/year n=17,815 | ||
| Procedure-Type Risk Group--no. (%) | < 0.001 | ||||
| Risk Group 1 | 1,550 (34.8) | 4,590 (35.9) | 8,649 (40.4) | 8,490 (47.7) | |
| Risk Group 2 | 1,883 (42.2) | 4,881 (38.2) | 7,011 (32.8) | 4,562 (25.6) | |
| Risk Group 3 | 766 (17.2) | 2,466 (19.3) | 4,250 (19.9) | 3,391 (19.0) | |
| Risk Group 4 | 261 (5.9) | 850 (6.6) | 1,481 (6.9) | 1,372 (7.7) | |
| Procedure Status--no. (%)* | < 0.001 | ||||
| Elective | 3,906 (87.8) | 11,228 (88.6) | 18,245 (85.8) | 14,797 (83.3) | |
| Urgent | 480 (10.8) | 1,159 (9.1) | 2,355 (11.1) | 2,435 (13.7) | |
| Emergent | 61 (1.4) | 259 (2.0) | 593 (2.8) | 503 (2.8) | |
| Salvage | 2 (0.0) | 26 (0.2) | 64 (0.3) | 27 (0.2) | |
| Procedure Time-- mean ± s.d.† | 100.3 ± 61.6 | 96.5 ± 64.0 | 92.0 ± 64.5 | 84.8 ± 66.4 | < 0.001 |
| Anesthesiologist Present--no. (%)‡ | 3,437 (77.2) | 12,137 (95.1) | 20,247 (94.8) | 15,714 (88.3) | < 0.001 |
| Airway Management--no. (%) | < 0.001 | ||||
| Spontaneous Respirations | 1,153 (25.9) | 1,920 (15.0) | 2,690 (12.6) | 3,147 (17.7) | |
| Bag Mask Ventilation | 3 (0.1) | 165 (1.3) | 19 (0.1) | 28 (0.2) | |
| CPAP | 3 (0.1) | 4 (0.0) | 14 (0.1) | 21 (0.1) | |
| Laryngeal Mask Airway | 144(3.2) | 836 (6.5) | 1,330 (6.2) | 1,361 (7.6) | |
| Elective Intubation at case start | 2,931 (65.7) | 9,193 (71.9) | 15,598 (72.9) | 11,315 (63.5) | |
| Tracheostomy | 45 (1.0) | 165 (1.3) | 223 (1.0) | 335 (1.9) | |
| Previously Intubated | 199 (4.5) | 840 (6.6) | 1,497 (7.0) | 1,632 (9.2) | |
Abbreviations: no., number; s.d., standard deviation; CPAP, continuous positive airway pressure
313 patients (11 from small, 115 from medium, 134 from large, 53 from very large volume programs) with missing information for procedure status
279 patients (26 from small, 72 from medium, 74 from large, 107 from very large volume programs) with missing information for procedure time
96 patients (7 from small, 27 from medium, 42 from large, 20 from very large volume programs) with missing information for Anesthesiologist present
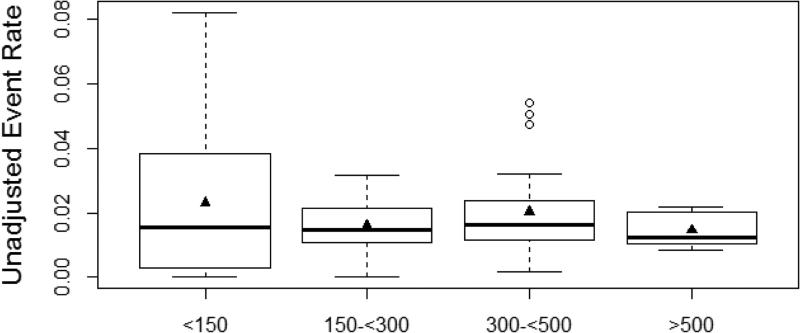
Overall, there were 1,014 major adverse events, with an overall rate of 1.8%. The most common adverse events were cardiac arrest (0.7%), unplanned cardiac surgery (0.4%), and subsequent cardiac catheterization due to a complication during the initial cardiac catheterization procedure (0.4%). In unadjusted analysis, major adverse events differed based upon annual institutional volume, with a major adverse event occurring in 123 (2.8%) cases at low-volume hospitals, 198 (1.5%) cases at medium-volume hospitals, 431 (2.0%) cases at high-volume hospitals, and 262 (1.5%) cases at very high-volume hospitals (p<0.001) (Table 4)(Figure 2).
Table 4.
Frequency of adverse events stratified by annual hospital volume
| Hospital Annual Volume | p-value | ||||
|---|---|---|---|---|---|
| <150 cases/year n=4,460 | ≥150-300 cases/year n=12,787 | ≥300-500 cases/year n=21,391 | ≥500 cases/year n=17,815 | ||
| Major Adverse Events--no. (%) | 123 (2.8) | 198 (1.5) | 431 (2.0) | 262 (1.5) | < 0.001 |
| Cardiac Arrest* | 41 (0.9) | 66 (0.5) | 155 (0.7) | 136 (0.8) | 0.02 |
| Arrhythmia (requiring PPM)† | 4 (0.1) | 1 (0.0) | 6 (0.0) | 5 (0.0) | 0.05 |
| Tamponade‡ | 11 (0.2) | 7 (0.1) | 28 (0.1) | 24 (0.1) | 0.01 |
| Air Embolus§ | 1 (0.0) | 4 (0.0) | 7 (0.0) | 8 (0.0) | 0.86 |
| Embolic Stroke∥ | 5 (0.1) | 7 (0.1) | 16 (0.1) | 4 (0.0) | 0.06 |
| New Need for Dialysis¶ | 8 (0.2) | 2 (0.0) | 25 (0.1) | 7 (0.0) | < 0.001 |
| Event Requiring ECMO# | 12 (0.3) | 22 (0.2) | 57 (0.3) | 45 (0.3) | 0.34 |
| Event Requiring LVAD** | 2 (0.0) | 2 (0.0) | 5 (0.0) | 2 (0.0) | 0.50 |
| Unplanned Cardiac Surgery†† | 36 (0.8) | 54 (0.4) | 114 (0.5) | 32 (0.2) | < 0.001 |
| Unplanned Vascular Surgery‡‡ | 9 (0.2) | 12 (0.1) | 20 (0.1) | 11 (0.1) | 0.05 |
| Unplanned Other Surgery§§ | 2 (0.0) | 3 (0.0) | 10 (0.0) | 4 (0.0) | 0.51 |
| Subsequent Cardiac Cath.∥∥ | 17 (0.4) | 60 (0.5) | 112 (0.5) | 38 (0.2) | < 0.001 |
Note: Individual adverse events do not add up to total adverse events because more than one adverse event could occur during a cath. lab visit
Abbreviations: no., number; PPM, permanent pacemaker; ECMO, extracorporeal membrane oxygenation; LVAD, left ventricular assist device; cath, catheterization
2 patients (1 from large, 1 from very large volume programs) with missing information for cardiac arrest
5 patients (1 from small, 2 from large, 2 from very large volume programs) with missing information for need for permanent pacemaker
4 patients (2 from large, 2 from very large volume programs) with missing information for tamponade
4 patients (2 from large, 2 from very large volume programs) with missing information for air embolus
5 patients (2 from large,3 from very large volume programs) with missing information for embolic stroke
4 patients (2 from large, 2 from very large volume programs) with missing information for new requirement for dialysis
4 patients (2 from large, 2 from very large volume programs) with missing information for need for ECMO
4 patients (2 from large, 2 from very large volume programs) with missing information for need for LVAD
3 patients (1 from small, 1 from large, 1 from very large volume programs) with missing information for need for cardiac surgery
4 patients (1 from small, 1 from large, 2 from very large volume programs) with missing information for need for vascular surgery
6 (1 from small, 2 from large, 3 from very large volume programs) with missing information for need for other surgery
6 patients (1 from small, 2 from large, 3 from very large volume programs) with missing information for need for subsequent cardiac cath.
Figure 2.
Unadjusted major adverse event rate (excluding death) based upon annual catheterization volume. Triangle represents mean and dark bar represents median rate. Adverse event rates are lower at medium, high, and very-high volume centers although variability within center volume exists, particularly for low-volume centers.
After adjusting for patient and procedural characteristics, the difference in rates of major adverse events by hospital procedural volume persisted. Compared with low-volume centers, the odds of an adverse event was 0.55 (95% confidence interval [CI]: 0.35, 0.86, p=0.008), 0.62 (95% CI: 0.41, 0.95, p=0.03), and 0.52 (95% CI: 0.31, 0.90, p=0.02) at medium-, high-, and very high-volume centers, respectively (Table 5). To further investigate the relationship between annual hospital volume and occurrence of a major adverse event, annual hospital volume was also treated as a continuous variable. Testing for a non-linear relationship using a 3-knot spline term was non-significant (p=0.96), therefore annual volume was modeled as a linear term. The relationship between annual hospital volume and occurrence of a major adverse event when modeling annual volume as a continuous variable became non-significant (odds ratio 0.94 [95% CI=0.88, 1.02] per increase in annual volume of 100 patients, p=0.13). A procedure-type risk group and hospital annual volume interaction demonstrated that the relationship between annual volume and occurrence of adverse events was no different for low-risk as compared with high-risk procedures (p=0.53). Likewise, an age and hospital annual volume interaction demonstrated that the relationship between annual volume and occurrence of adverse events was no different for adults (≥18 years of age) as compared with non-adults (<18 years of age) (p=0.87).
Table 5.
Adjusted Risk of Major Adverse Event
| Primary Analysis (not including death) | Secondary Analysis (including death) | |||
|---|---|---|---|---|
| Predictor | Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value |
| Annual Volume | ||||
| <150 | Reference | Reference | ||
| 150-<300 | 0.55 (0.35, 0.86) | 0.008 | 0.82 (0.59, 1.14) | 0.24 |
| 300-<500 | 0.62 (0.41, 0.95) | 0.03 | 0.73 (0.53, 1.01) | 0.06 |
| ≥500 | 0.52 (0.31, 0.90) | 0.02 | 0.78 (0.52, 1.15) | 0.21 |
| Procedure-type risk group | ||||
| 1 | Reference | Reference | ||
| 2 | 1.31 (1.06, 1.62) | 0.01 | 1.86 (1.44, 2.39) | <0.001 |
| 3 | 1.57 (1.26, 1.96) | <0.001 | 1.76 (1.35, 2.31) | <0.001 |
| 4 | 2.03 (1.59, 2.61) | <0.001 | 2.03 (1.50, 2.77) | <0.001 |
| Age | ||||
| <30 days | Reference | Reference | ||
| ≥30 days to ≤1 year | 0.98 (0.77, 1.25) | 0.89 | 0.89 (0.71, 1.11) | 0.31 |
| 1≤18 years | 0.86 (0.66, 1.11) | 0.24 | 0.69 (0.55, 0.88) | 0.002 |
| >18 years | 0.94 (0.69, 1.29) | 0.72 | 0.82 (0.62, 1.08) | 0.16 |
| Genetic/Congenital condition | 1.05 (0.86, 1.28) | 0.64 | 1.36 (1.15, 1.59) | <0.001 |
| History of CLD | 0.86 (0.67, 1.12) | 0.27 | 1.79 (1.49, 2.15) | <0.001 |
| History of Renal Insufficiency | 2.32 (1.77, 3.04) | <0.001 | 3.16 (2.50, 4.01) | <0.001 |
| Single Ventricle | 1.34 (1.15, 1.56) | <0.001 | 1.39 (1.22, 1.60) | <0.001 |
| Pre-procedure sepsis | 1.43 (0.88, 2.31) | 0.15 | 1.50 (0.95, 2.38) | 0.08 |
| Need for Inotropes | 1.75 (1.43, 2.14) | <0.001 | 2.58 (2.16, 3.08) | <0.001 |
| Need for ECMO | 1.09 (0.77, 1.54) | 0.63 | 5.79 (4.22, 7.93) | <0.001 |
| Need for LVAD | 0.81 (0.19, 3.43) | 0.77 | 2.97 (0.96, 9.25) | 0.06 |
| Procedure Status | ||||
| Elective | Reference | Reference | ||
| Urgent | 2.14 (1.78, 2.58) | <0.001 | 2.99 (2.55, 3.50) | <0.001 |
| Emergent | 3.51 (2.69, 4.57) | <0.001 | 4.42 (3.47, 5.63) | <0.001 |
| Salvage | 5.85 (3.37, 10.14) | <0.001 | 21.80 (11.95, 39.79) | <0.001 |
| Saturation <95% (non-SV) or <78% (SV) | ||||
| No | Reference | Reference | ||
| Yes | 1.13(0.96, 1.33) | 0.15 | 1.13 (0.98, 1.31) | 0.08 |
| Missing | 1.11 (0.87, 1.41) | 0.39 | 1.00 (0.80, 1.26) | 0.97 |
| MV Saturation <60% (non-SV) or <50% (SV) | ||||
| No | Reference | Reference | ||
| Yes | 1.80 (1.49, 2.18) | <0.001 | 1.85 (1.58, 2.18) | <0.001 |
| Missing | 1.31 (1.04, 1.64) | 0.02 | 1.65 (1.35, 2.02) | <0.001 |
| MPA Systolic Pressure ≥45 (non-SV) or MPA Mean Pressure ≥17mmHg (SV) | ||||
| No | Reference | Reference | ||
| Yes | 1.32 (1.09, 1.59) | 0.004 | 1.99 (1.71, 2.32) | <0.001 |
| Missing | 1.42 (1.18, 1.71) | <0.001 | 1.27 (1.07, 1.51) | 0.005 |
| Systemic Ventricular EDP ≥ 18mmHg | ||||
| No | Reference | Reference | ||
| Yes | 1.48 (1.14, 1.94) | 0.004 | 1.49 (1.18, 1.87) | <0.001 |
| Missing | 1.21 (1.04, 1.41) | 0.02 | 1.13 (0.99, 1.30) | 0.07 |
Abbreviations:CI, confidence interval; CLD, chronic lung disease; ECMO, extracorporeal membrane oxygenation; LVAD, left ventricular assist device; SV, single ventricle; MV, mixed venous; MPA, main pulmonary artery; EDP, end diastolic pressure
As a secondary analysis, the association between annual hospital volume and occurrence of an adverse event was evaluated with in-hospital death included in the composite outcome. The overall rate of death during the index hospitalization was 1.5% (800/52,295 episodes of care), and this rate was similar at low-volume (61/4,332 [1.4%]), medium volume (185/11,974 [1.5%]), high-volume (315/19,882 [1.6%]), and very high-volume centers (239/16,107 [1.5%]; p-value for difference across the 4 hospital volume categories of 0.79). In unadjusted analysis, the rate of major adverse events (including death) differed by annual institutional volume: low-volume centers (153/4,332; 3.5%), medium-volume centers (353/11,974; 2.9%), high-volume centers (556/19,982; 2.8%), and very high-volume centers (410/16,107; 2.5%) (p=0.004). No association was observed between annual hospital procedural volume and occurrence of an adverse event (when death was included) after multivariable adjustment regardless of whether volume was treated as a categorical (p=0.29) (Table 4) or continuous variable (odds ratio 0.96 [95% CI=0.92, 1.01] per increase in annual volume of 100, p=0.16)
Discussion
In a large multicenter registry of cardiac procedures among pediatric and adult patients with CHD, we found that cardiac catheterization procedures were generally safe with overall peri-procedural rates of major adverse events of less than 2%. Despite the low rate, the adjusted odds of a major adverse event peri-procedurally, when excluding death, were 38% to 48% lower at hospitals with annual case volumes exceeding 150 cardiac catheterization procedures. While the absolute differences were small, these data suggest a small volume-outcome relationship for cardiac catheterization procedures among pediatric and adult patients with CHD, although this relationship was not observed when death was included as a major adverse event.
The concept of a “volume-outcome” relationship has been evaluated in several clinical settings, including cardiovascular procedures. For instance, a prior study of complications related to ICD implantation has reported an inverse relationship between occurrence of an adverse event and annual procedural volume(1). A similar inverse relationship was found between hospital procedure volume and outcomes following percutaneous coronary intervention(2). Within the pediatric literature, a relationship has been demonstrated for cardiac surgery with higher peri-procedural mortality at hospitals performing fewer cardiac surgeries(3-10). Interestingly, this relationship seems to be most relevant for high-complexity procedures(3).
Studies on the relationship between procedural volume and adverse events for patients with CHD undergoing cardiac catheterization, on the other hand, have been few. In a study using data from Congenital Cardiac Catheterization Project on Outcomes (C3PO), the authors found that operators with less overall experience (<5 years in practice) were more likely to experience an adverse event compared with operators with 5-25 years in practice (11). In a more recent study involving data on cardiac catheterization procedures within the Pediatric Health Information Systems (PHIS) database, an inverse relationship between annual institutional volume and risk of death or need for mechanical circulatory support in the immediate post-catheterization period was reported(12). While an important finding, this study was performed using administrative data with limited detail about patient- (e.g. hemodynamic data) and procedure-level (e.g. specific interventional procedure) factors, which limits the degree to which risk stratification could be performed. Additionally, the ability to include more granular data regarding procedural outcomes was lacking, given the administrative data source.
A relationship between hospital volume and outcomes has important implications within the health care system. If higher-volume hospitals do, in fact, have improved outcomes, patients with CHD may be able to make more informed decisions about where to obtain invasive diagnostic or interventional cardiac procedures. Organizations such as the Leapfrog group promote the transparent reporting of quality and outcomes within the healthcare system and have proposed creating volume-standards for certain types of high-risk surgical procedures (18). Our study is one of the first to describe the relationship between volume and outcomes for congenital cardiac catheterization and suggests that the risk of cardiac catheterization for pediatric and adult patients with CHD may be slightly higher at centers performing fewer than 150 procedures annually. Interestingly, this same institutional procedural volume threshold has been previously put forth in a number of opinion-based consensus statements related to performance of higher complexity procedures and training in pediatric cardiac catheterization(19-21).
In our study, medium-, high-, and very high-volume centers were less likely to experience a major adverse event as compared with low-volume centers. However, despite this statistically significant categorical association between volume and outcomes, the low event rate translates into small absolute differences in rates of major adverse events between low-volume and higher-volume centers. Additionally, we see a significant amount of variability in risk even within volume categories (particularly among low-volume centers), indicating that center volume alone does not explain all of the variability in outcomes between institutions. For these reasons, it would be difficult to make definitive recommendations regarding regionalization of care for these procedures based solely on our study findings. Rather, the findings of our study should be considered along with other factors, including patient need and preference. Patients with CHD in need of a cardiac catheterization may prefer to seek care closer to home, particularly if they can do so with only a small increased risk from local treatment. Additionally, selective referral to high-volume centers can create unnecessary barriers to care for patients with limited resources needing to travel to a higher-volume center and can create delays in care for patients in need of immediate treatment. Without a more robust difference in outcome based upon center volume, each of these factors must be carefully considered before selectively referring a patient to another hospital based solely on center volume. Furthermore, while we found a small difference in outcomes based upon center volume, we were unable to determine the reason for lower rates of major adverse events at higher volume centers. While it is possible that improved outcomes are an inextricable benefit of more procedural experience, it is also possible that there are learned best practices at higher volume centers. If these best practices could be transmitted to all centers, it could also obviate the need for selective referral based upon center volume.
Despite finding a significant categorical association between volume and outcomes, we did not find a statistically significant relationship between volume and outcomes when modeling volume continuously. Intuitively, this finding makes sense as the volume-outcome relationship is unlikely to be linear, at least not along all points of the volume spectrum. As an example, it is likely that increasing annual center volume from 25 to 125 procedures would result in a larger effect on patient outcomes than an increase in annual center volume from 1000 to 1100 procedures, even though this difference would be treated the same in a linear model of center volume. Instead, our results suggest that there may be a “critical mass” needed in order to achieve similar outcomes. In fact, when comparing outcomes between small volume programs (<150 cases/year) and all other centers, we found that centers performing more than 150 cases annually were less likely to experience an adverse event (OR 0.57, 95% CI 0.40, 0.84, p=0.004).
Additionally, in our study, we did not find a significant relationship between volume and outcomes in our secondary analysis, when in-hospital death was included as a component of the composite end point. It is important to note that the definition of “death” in the current version of IMPACT includes death that occurred at any time following cardiac catheterization and prior to hospital discharge, without adjudication regarding attribution to the catheterization. As such, mortality events may represent the illness severity of patients and may not be secondary to their cardiac catheterization procedure. Indeed, a prior study found that 30-day mortality in patients with CHD undergoing cardiac catheterization is unlikely to be related to the cardiac catheterization procedure but rather to pre-procedural morbidity, subsequent in-hospital events, or non-cardiac causes(15). Furthermore, in the prior study evaluating the relationship between volume and outcomes in the PHIS database, the association between center volume and risk of catastrophic adverse events was evident in the immediate post-catheterization period, however, when the timeline for follow-up was extended until hospital discharge, the relationship between volume and outcomes was less clear. The lack of association between volume and outcomes when death is included in the composite end point, in our study, suggests that death within a certain timeframe following catheterization or death with some adjudication regarding cause, may be the most suitable marker of catheterization-related quality of care.
Our findings should be interpreted in the context of the following potential limitations. First, we did not evaluate the relationship between individual operator volume and adverse events. Institutional volume and operator volume might be independently related to adverse event rates but are also likely to be collinear. This complicated relationships will need to be evaluated in future studies. Second, while we presumed that the occurrence of a major adverse event was directly related to the cardiac catheterization, we cannot rule out the possibility that the adverse event may have been unrelated to the catheterization procedure. This is particularly germane to the occurrence of death, which we presumed was not related to the percutaneous procedure and included it only as a secondary analysis. Future data collection efforts for IMPACT should seek to discriminate between deaths attributable to cardiac catheterization procedures and other complications of care. Third, while some institutions may restrict coding of major adverse events to complications that are directly linked to the cardiac catheterization, other institutions may code any in-hospital adverse event, regardless of direct linkage to the catheterization. Differences in reporting of complication rates among hospitals could thus have influenced our study findings, especially if they systematically varied by hospital volume. Given that IMPACT is a relatively new registry with no publicly available data audits, results from future data audits should be evaluated to ensure that data reporting is consistent between institutions and that institutions are appropriately attributing adverse events during the data reporting process. Lastly, our study was an observational study and is subject to the same limitations as all observational studies, including the possibility of unmeasured confounding. For example, low-volume centers had a higher proportion of adult patients. While we adjusted for age in our multivariable analysis, there may be other characteristics unique to adults that we did not adjust for.
Conclusions
Using a large multicenter registry, we found that centers performing at least 150 cardiac catheterizations on pediatric and adult patients with CHD had a numerically small but statistically significantly lower rate of adverse events as compared with low-volume centers. Our findings provide some support for regionalizing care at centers performing more than 150 cases per year, but other issues of access to care and patient preference will also need to be considered.
Acknowledgments
Funding/Support:
Dr. Jayaram was supported by a T32 training grant (HL110837) from the National Heart Lung and Blood Institute.
Dr. O'Byrne received support from the NIH [T32 HL007915] and Entelligence Young Investigator grant
Dr. Spertus is the PI of the analytic center that is under contract with the American College of Cardiology Foundation to analyze the NCDR.
Dr. Chan is supported by an R01 Award (1R01HL123980) from the National Heart Lung and Blood Institute.
Abbreviations
- ICD
implantable cardioverter defibrillator
- CHD
congenital heart disease
- NCDR
National Cardiovascular Data Registry
- IMPACT
Improving Pediatric and Adult Congenital Treatment
- ECMO
Extracorporeal membrane oxygenation
- LVAD
Left Ventricular Assist Device
- C3PO
Congenital Cardiac Catheterization Project on Outcomes
- CI
confidence interval
- STS
Society for Thoracic Surgeons
- PHIS
Pediatric Health Information System
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Dr. Spertus discloses grant funding from NIH, AHA, Lilly, Gillead, Amorcyte and Genentech. He serves on Scientific Advisory Boards for United Healthcare, Novartis, Regeneron and Amgen. He serves as a paid editor for Circulation: Cardiovascular Quality and Outcomes and has intellectual property rights for the Seattle Angina Questionnaire, Kansas City Cardiomyopathy Questionnaire, Peripheral Artery Questionnaire and an equity interest in Health Outcomes Sciences
- Dr. Glatz is a consultant for Bristol-Myers Squibb and the Medicines Company
- Dr. Bergersen is a consultant for 480 Biomedical Inc.
REFERENCES
- 1.Freeman JV, Wang Y, Curtis JP, Heidenreich PA, Hlatky MA. The relation between hospital procedure volume and complications of cardioverter-defibrillator implantation from the implantable cardioverter-defibrillator registry. J Am Coll of Cardiol. 2010;56:1133–9. doi: 10.1016/j.jacc.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 2.McGrath PD, Wennberg DE, Dickens JD, Jr., et al. Relation between operator and hospital volume and outcomes following percutaneous coronary interventions in the era of the coronary stent. JAMA : the Journal of the American Medical Association. 2000;284:3139–44. doi: 10.1001/jama.284.24.3139. [DOI] [PubMed] [Google Scholar]
- 3.Welke KF, O'Brien SM, Peterson ED, Ungerleider RM, Jacobs ML, Jacobs JP. The complex relationship between pediatric cardiac surgical case volumes and mortality rates in a national clinical database. The Journal of Thoracic and Cardiovascular Surgery. 2009;137:1133–40. doi: 10.1016/j.jtcvs.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Karamlou T, Jacobs ML, Pasquali S, et al. Surgeon and center volume influence on outcomes after arterial switch operation: analysis of the STS Congenital Heart Surgery Database. The Annals of Thoracic Surgery. 2014;98:904–11. doi: 10.1016/j.athoracsur.2014.04.093. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch JC, Gurney JG, Donohue JE, Gebremariam A, Bove EL, Ohye RG. Hospital mortality for Norwood and arterial switch operations as a function of institutional volume. Pediatric cardiology. 2008;29:713–7. doi: 10.1007/s00246-007-9171-2. [DOI] [PubMed] [Google Scholar]
- 6.Pasquali SK, Li JS, Burstein DS, et al. Association of center volume with mortality and complications in pediatric heart surgery. Pediatrics. 2012;129:e370–6. doi: 10.1542/peds.2011-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannan EL, Racz M, Kavey RE, Quaegebeur JM, Williams R. Pediatric cardiac surgery: the effect of hospital and surgeon volume on in-hospital mortality. Pediatrics. 1998;101:963–9. doi: 10.1542/peds.101.6.963. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins KJ, Newburger JW, Lock JE, Davis RB, Coffman GA, Iezzoni LI. In-hospital mortality for surgical repair of congenital heart defects: preliminary observations of variation by hospital caseload. Pediatrics. 1995;95:323–30. [PubMed] [Google Scholar]
- 9.Bazzani LG, Marcin JP. Case volume and mortality in pediatric cardiac surgery patients in California, 1998-2003. Circulation. 2007;115:2652–9. doi: 10.1161/CIRCULATIONAHA.106.678904. [DOI] [PubMed] [Google Scholar]
- 10.Checchia PA, McCollegan J, Daher N, Kolovos N, Levy F, Markovitz B. The effect of surgical case volume on outcome after the Norwood procedure. The Journal of Thoracic and Cardiovascular Surgery. 2005;129:754–9. doi: 10.1016/j.jtcvs.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 11.Holzer RJ, Gauvreau K, Kreutzer J, Moore JW, McElhinney DB, Bergersen L. Relationship between procedural adverse events associated with cardiac catheterization for congenital heart disease and operator factors: Results of a multi-institutional registry (C3PO). Catheterization and cardiovascular interventions : Official Journal of the Society for Cardiac Angiography & Interventions. 2013 doi: 10.1002/ccd.24866. [DOI] [PubMed] [Google Scholar]
- 12.O'Byrne ML, Glatz AC, Shinohara RT, et al. Effect of center catheterization volume on risk of catastrophic adverse event after cardiac catheterization in children. American Heart Journal. 2015;169:823–832. e5. doi: 10.1016/j.ahj.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin GR, Beekman RH, Ing FF, et al. The IMPACT registry: IMproving Pediatric and Adult Congenital Treatments. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13:20–5. doi: 10.1053/j.pcsu.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Bergersen L, Gauvreau K, Marshall A, et al. Procedure-type risk categories for pediatric and congenital cardiac catheterization. Circulation Cardiovascular Interventions. 2011;4:188–94. doi: 10.1161/CIRCINTERVENTIONS.110.959262. [DOI] [PubMed] [Google Scholar]
- 15.Backes CH, Bergersen L, Rome JJ, et al. Quality metrics in cardiac catheterization for congenital heart disease: utility of 30-day mortality. Catheterization and Cardiovascular Interventions : Official Journal of the Society for Cardiac Angiography & Interventions. 2015;85:104–10. doi: 10.1002/ccd.25683. [DOI] [PubMed] [Google Scholar]
- 16.Bergersen L, Gauvreau K, Foerster SR, et al. Catheterization for Congenital Heart Disease Adjustment for Risk Method (CHARM). J Am Coll Cardiol Intv. 2011;4:1037–46. doi: 10.1016/j.jcin.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 17.R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing V; Austria: 2008. ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- 18.Birkmeyer JD, Finlayson EV, Birkmeyer CM. Volume standards for high-risk surgical procedures: potential benefits of the Leapfrog initiative. Surgery. 2001;130:415–22. doi: 10.1067/msy.2001.117139. [DOI] [PubMed] [Google Scholar]
- 19.Armsby LB, Vincent RN, Foerster SR, et al. Task Force 3: Pediatric Cardiology Fellowship Training in Cardiac Catheterization. J Am Coll Cardiol. 2015;66:699–705. doi: 10.1016/j.jacc.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Hijazi ZM, Ruiz CE, Zahn E, et al. SCAI/AATS/ACC/STS operator and institutional requirements for transcatheter valve repair and replacement, Part III: Pulmonic valve. Catheterization and Cardiovascular Interventions : Official Journal of the Society for Cardiac Angiography & Interventions. 2015;86:85–93. doi: 10.1002/ccd.25710. [DOI] [PubMed] [Google Scholar]
- 21.Bashore TM, Balter S, Barac A, et al. 2012 American College of Cardiology Foundation/Society for Cardiovascular Angiography and Interventions expert consensus document on cardiac catheterization laboratory standards update: A report of the American College of Cardiology Foundation Task Force on Expert Consensus documents developed in collaboration with the Society of Thoracic Surgeons and Society for Vascular Medicine. J Am Coll Cardiol. 2012;59:2221–305. doi: 10.1016/j.jacc.2012.02.010. [DOI] [PubMed] [Google Scholar]