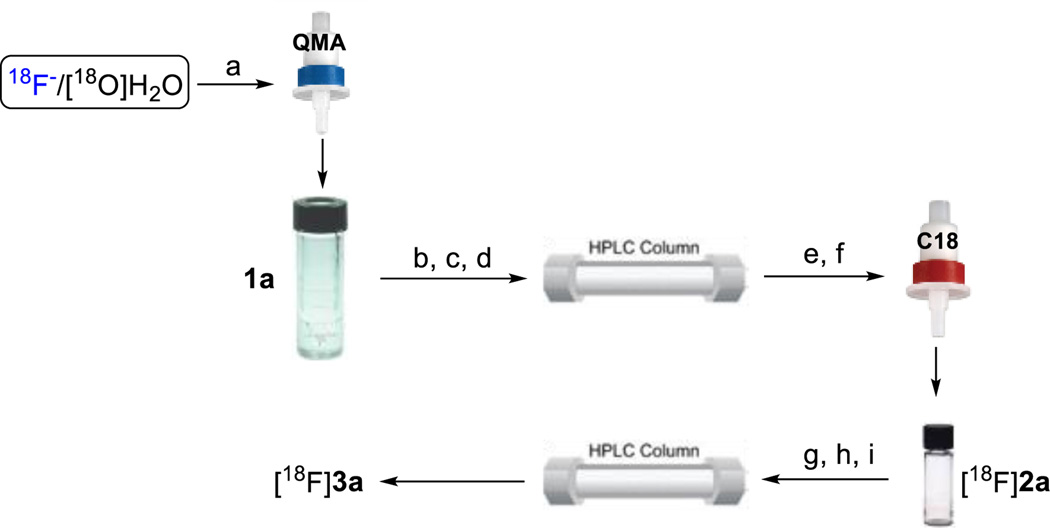
Scheme 3.
Synthesis of [18F]2a and [18F]3a. Synthesis of [18F]2a: (a) trap and release 18F− into V-vial via a solution of TEAB in 1 mL MeCN/H2O (v/v 7:3); (b) azeotropic drying with anhydrous MeCN (3 × 1 mL, 3 min for each cycle); (c) addition of 1a, MeCN, 130°C, 10 min; (d) dilution and subsequent purification of the reaction mixture (~12 min); (e) concentration of the eluent via C18 cartridge (~2 min); (f) release of [18F]2a via 1 mL MeCN(~1 min). Synthesis of [18F]3a: (g) addition of Na2S2O8, Selectfluor and EtOH(~1 min); (h) 120°C, 10 min; (i) dilution and subsequent purification of the reaction mixture (~12 min).