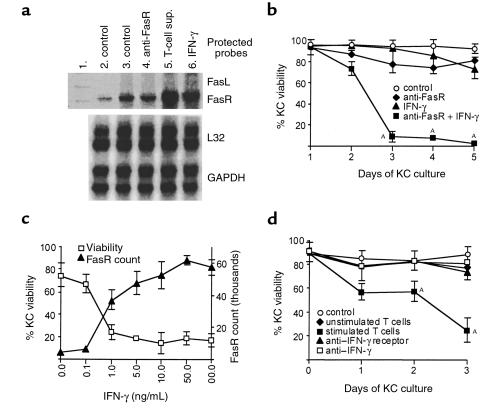
Figure 2.
(a) Expression of FasR mRNA by primary human KCs. Lane 1: unprotected template. Lane 2: after 8 hours of KC culture. Lane 3: after 24 hours of KC culture. Lane 4: after 16 hours of KC culture followed by 8 hours with 1.0 μg/mL anti-FasR mAb. Lane 5: after 16 hours of KC culture followed by 8 hours with diluted (50%) supernatant (sup.) from stimulated CD45RO+ T cells. Lane 6: after 16 hours of KC culture followed by 8 hours with 1.0 ng/mL IFN-γ. A representative result of three experiments is shown. (b) IFN-γ and Fas-induced KC apoptosis. KC viability was monitored by ethidium bromide exclusion and flow cytometry. Treatments shown are control (KCs alone), 1 μg/mL activating anti-FasR mAb, 10 ng/mL IFN-γ, and 1 μg/mL anti-FasR mAb added 1 day after starting incubation with 10 ng/mL IFN-γ. AP < 0.05. (c) IFN-γ–induced FasR counts exhibit a threshold for KC apoptosis. KCs were pretreated with the indicated doses of IFN-γ; 1 μg/mL anti-FasR mAb was added 1 day after starting incubation with IFN-γ. KC viability was assessed by ethidium bromide exclusion and flow cytometry at day 3. FasR count 1 day after starting incubation with indicated doses of IFN-γ. (d) CD45RO+ T cell–induced KC death is inhibited by blocking IFN-γ. KC viability was monitored by ethidium bromide exclusion and flow cytometry. In the flow cytometry setting, KCs and T cells are gated according to forward and side scatter. Both cell populations were therefore monitored separately. Coculture of primary human KCs and autologous unstimulated or stimulated (with anti-CD2, anti-CD3, and anti-CD28 mAb) CD45RO+ T cells. AP < 0.05. Inhibition of CD45RO+ T cell–induced KC death by 1 μg/mL blocking anti–IFN-γ receptor mAb and 20 μg/mL neutralizing anti–IFN-γ mAb. Results in b–d represent mean ± SD of triplicate cultures from three different experiments. Control, KCs alone.