Abstract
It is unknown whether a scaffold containing both small intestinal submucosa (SIS) and mesenchymal stem cells (MSCs) for transplantation may improve pancreatic islet function and survival. In this study, we examined the effects of a SIS-MSC scaffold on islet function and survival in vitro and in vivo. MSCs and pancreatic islets were isolated from Sprague-Dawley rats, and SIS was isolated from Bamei pigs. The islets were apportioned among 3 experimental groups as follows: SIS-islets, SIS-MSC-islets and control-islets. In vitro, islet function was measured by a glucose-stimulated insulin secretion test; cytokines in cultured supernatants were assessed by enzyme-linked immunosorbent assay; and gene expression was analyzed by reverse transcription-quantitative PCR. In vivo, islet transplantation was performed in rats, and graft function and survival were monitored by measuring the blood glucose levels. In vitro, the SIS-MSC scaffold was associated with improved islet viability and enhanced insulin secretion compared with the controls, as well as with the increased the expression of insulin 1 (Ins1), pancreatic and duodenal homeobox 1 (Pdx1), platelet endothelial cell adhesion molecule 1 [Pecam1; also known as cluster of differentiation 31 (CD31)] and vascular endothelial growth factor A (Vegfa) in the islets, increased growth factor secretion, and decreased tumor necrosis factor (TNF) secretion. In vivo, the SIS-MSC scaffold was associated with improved islet function and graft survival compared with the SIS and control groups. On the whole, our findings demonstrate that the SIS-MSC scaffold significantly improved pancreatic islet function and survival in vitro and in vivo. This improvement may be associated with the upregulation of insulin expression, the improvement of islet microcirculation and the secretion of cytokines.
Keywords: mesenchymal stem cells, small intestinal submucosa, pancreatic islet, scaffold, diabetes
Introduction
Diabetes poses a significant global health concern. It is estimated that 382 million individuals are suffering from diabetes, and this number is expected to increase to 592 million by the year 2035 (1). Although pancreatic islet transplantation has been proposed as an effective therapy in diabetes, it is largely limited by the shortage of islet donors, poor islet survival and the requirement for lifelong immunosuppression (2). In recent years, biomaterials have been used as an immunoisolation technique in islet transplantation. This technique aims at producing biological barriers which prevent immune cell migration and maintain the long-term function of transplanted islets (3,4). Biomaterials putatively offer several potential benefits, such as delivering proteins and growth factors, protecting the islets from immune rejection without the use of an immunosuppressor, and increasing the safety and clinical effect of the procedure (5–7). A number of natural and synthetic materials have been investigated in islet transplantation, including alginate, polyvinyl alcohol and silk hydrogel (8–10). However, there are still significant challenges that need to be resolved, such as methods of providing oxygen and nutrients to coated islets, reducing the damage due to the inflammatory response, and selecting suitable sites for transplantation (11).
The porcine small intestinal submucosa (SIS) is a new bioactive material composed of collagen I and fibronectin. SIS includes proteoglycan, glycosaminoglycan, glycoprotein and growth factors (12). Compared with other synthetic materials, SIS is easy to handle and elicits no immune response in the recipient organism (13). As a safe material, SIS has been successfully applied in clinical practice, including general pediatric surgery, urology and neurosurgery (14). SIS has a 3-dimensional microarchitecture that contains many cytokines, and can serve as a scaffold for cell growth and proliferation (15,16). Several research groups, including ours, have demonstrated that SIS can improve the islet survival rate, boost insulin secretion and reduce cell apoptosis in vitro (17–19); however, the precise mechanisms and the effects in vivo remain unclear.
The mesenchymal stromal cell (MSC) is an adult stem cell (20) that is considered a suitable candidate for regenerative medicine and cell-based therapy, due to its ease of isolation, self-renewal potential, multipotency and immunomodulatory function (21,22). MSCs can promote angiogenesis by producing a large number of cytokines. In addition, MSCs have anti-apoptotic, anti-inflammatory and mitogenic effects. It has been reported that MSCs can maintain islet organization and morphology, improve graft revascularization, suppress inflammatory damage and mediate immune responses, promoting prolonged graft survival and enhanced islet function (23–27).
It is unknown whether a scaffold containing both SIS and MSCs may improve islet function and islet survival. Thus, in the present study, in an aim to clarify this issue, we investigated the effects of a SIS-MSC scaffold on islet function and survival in vitro and in vivo.
Materials and methods
Rats
The Animal Care and Use Committee of Xi'an Jiaotong University approved all the animal protocols. Sprague-Dawley rats were purchased from the Laboratory Animal Center, Xi'an Jiaotong University, Xi'an, China. Bamei pigs were purchased from Xi'an Zhuque market, Xi'an, China. Rats (Laboratory Animal Center, Xi'an Jiaotong University, Xi'an, China) were raised in a specific-pathogen-free laboratory (temperature 18–26°C, relative humidity 40–70%) and were provided with free access to food and water.
Rat MSC isolation and identification
MSCs were acquired from Sprague-Dawley rats (n=8; male, 3 weeks of age, 60–80 g). The rats were sacrificed by the spinal dislocation method before obtaining the bones. Briefly, the femurs and tibiae were removed in a sterile environment and the bone cavity was lightly flushed with phosphate-buffered saline (PBS) using a 21-gauge needle. The flushed samples were centrifuged at 1,000 rpm for 5 min, and the supernatant was removed. The cells were resuspended in complete medium (Cyagen Biosciences, Guangzhou, China), and incubated by the adherence culture method, as previously descrbied (28) to isolate the MSCs. MSCs were identified by surface molecular markers [CD90 (anti-mouse/rat CD90.1(Thy-1.1) PE; 12-0900-81, eBioscience, San Diego, CA USA) and CD34 (anti-mouse CD34 FITC; 11-0341-82, eBioscience)] with the use of a flow cytometer, and their ability to differentiate into osteoblast-like and adipocyte-like cells was assessed by Alizarin Red S and Oil Red O staining (both from Cyagen Biosciences). All experiments were carried out using MSCs at passage 3–6.
Isolation of SIS from Bamei pigs and SIS-MSC scaffold preparation
SIS isolation and preparation was performed as previously described (29). Briefly, Bamei pigs (male, 6 months of age, 100–120 kg) were sacrificed by a lethal injection of sodium pentobarbital and the SIS was prepared within 4 h. The jejunum was washed with water, and fat, tunica serosa, and tunica muscularis were removed. The jejunum was dipped in 1 g/l peroxyacetic acid (Shaanxi Three Bridge Chemical Co., Ltd., Shaanxi, China) to sterilize and maintained in PBS until use. SIS was identified by hematoxylin and eosin (H&E) staining and scanning electron microscopy (Hitachi, Tokyo, Japan).
To generate the SIS-MSC scaffold, the SIS was cut into 22×22 mm sections and each section was placed on a Nunclon 35-mm petri dish (Thermo Fisher Scientific, Waltham, MA, USA). Approximately 1×106 MSCs were seeded in the dish and cultured for 48 h to form a confluent monolayer.
Rat islet isolation and identification
Islets were isolated from Sprague-Dawley rats (n=10; male, 8 weeks of age, 280–300 g) as previously described (30). The rats were anesthetized by an intraperitoneal injection of pentobarbital before obtaining the pancreas for the islets. The pancreas was digested with 1 mg/ml collagenase P (Roche, Mannheim, Germany) and purified by Histopaque-1077 (Sigma, St. Louis, MO, USA). After washing with RPMI-1640 medium (Gibco, Carlsbad, CA, USA), the islets were distributed into groups of 200 for culture. Islets were identified by dithizone and their viability was estimated by acridine orange/propidium iodide (AO/PI), described below (both from Gibco).
Experimental design
The islets were divided into 3 groups as follows: group A, islets; group B, SIS-islets; and group C, SIS-MSC-islets. In the controls (group A), 200 fresh islets were cultured alone in non-treated 35-mm Petri dishes. For group B, 200 fresh islets were seeded on SIS. For group C, 200 fresh islets were seeded on the SIS-MSC scaffold.
RPMI-1640 containing 10% fetal calf serum was used for all co-culture configurations. All islets were cultured in a carbon dioxide incubator (37°C, 5% CO2).
In vitro islet function tests
The islets from the 3 groups were collected on days 7 and 14. The viability rate of the islets was estimated by AO/PI as follows: viability rate, % = numbersgreen/numbersgreen + red ×100.
Islet function was measured by a glucose-stimulated insulin secretion test. Briefly, the islets were incubated in RPMI-1640 containing 1.67 mmol/l glucose for 2 h, and then incubated in RPMI-1640 containing 16.7 mmol/l glucose for a further 2 h. Insulin secretion was assessed with an enzyme-linked immunosorbent assay (ELISA) kit (Mercodia, Uppsala, Sweden). The insulin release stimulation index (SI) was calculated as follows: SI = insulin concentration (16.7 mmol/l)/insulin concentration (1.67 mmol/l).
Immunohistological analysis and cytokine detection
In each group, the islets and cultured supernatants were obtained at day 7. The islets were fixed in 10% paraformaldehyde (Beyotime, Shanghai, China) overnight and embedded in paraffin. The paraffin sections were stained with a rabbit insulin monoclonal antibody (1:100; Cell Signaling Technology, Danvers, MA, USA), followed by 3,3′-diaminobenzidine (DAB) immunostaining. Images were acquired using a light microscope (Olympus, Tokyo, Japan). Cytokines [vascular endothelial growth factor A (VEGFA), ciliary neurotrophic factor (CNTF), epidermal growth factor (EGF), hepatocyte growth factor (HGF) and tumor necrosis factor (TNF)] in cultured supernatants were detected using an ELISA kit (Mercodia) in accordance with the manufacturer's instructions.
Reverse transcription-quantitative PCR (RT-qPCR)
RNA was extracted from the islets on day 14 using an RNeasy kit (Qiagen, Valencia, CA, USA). Reverse transcription was performed using a FastQuant RT kit with gDNase (Tiangen, Beijing, China) from 2 mg total RNA. Quantitative PCR (qPCR) was performed using a maxima SYBR-Green qPCR master mix (Thermo Fisher Scientific) protocol on the instructions of CFX96 (Bio-Rad, Hercules, CA, USA). The PCR products were amplified using the following primer sets: for glyceraldehyde 3-phosphate dehydrogenase (Gapdh), 5′-TACCCACGGCAAGTTCAACG-3′ and 5′-CACCAGCATCACCCCATTTG-3′; for pancreatic and duodenal homeobox 1 (Pdx1), 5′-GGAACGCTGGAACAGGGAAG-3′ and 5′-CAGTCTCGGTTCCATTCGGG-3′; for insulin 1 (Ins1), 5′-GACCATTGATTCCGTGACAT-3′ and 5′-CACCAGAGCATAGGAGCGAC-3′; and for vascular endothelial growth factor A (Vegfa), 5′-AGGAGTACCCCGATGAGATA-3′ and 5′-ATCTCTCCTATGTGCTGGCT-3′.
Every sample was tested in triplicate and the results were quantified using the Livak 2−ΔΔCT method, where CT is the cycle threshold. Relative gene expression was determined by the ΔΔCT method relative to the GAPDH gene expression. All data are presented as the means ± SEM. A value of P<0.05 was considered to indicate a statistically significant difference compared to the control group.
Immunofluorescence staining
The cultured islets were collected on day 14 and treated using a cell smear centrifuge. The smears were fixed in 4% paraformaldehyde and permeabilized with Triton X-100 (Beyotime). After blocking with 10% donkey serum, the smears were incubated serially with a rabbit insulin monoclonal antibody (#3014) or a mouse cluster of differentiation (CD)31 monoclonal antibody (#3528) (1:100; both from Cell Signaling Technology). The smears were then incubated with secondary antibody (donkey anti-rabbit Alexa 488, 711-545-152, 1:500 or donkey anti-mouse Cy3, 715-165-150, 1:500; Jackson Immunoresearch, West Grove, PA, USA). Finally, the smears ware counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Beyotime). Images were obtained using a confocal laser scanning microscope (Leica). Graphical representations are expressed as the average of mean fluorescence intensity (MFI) using arbitrary units (AU) ± SD. A value of P<0.05 was considered to indicate a statistically significant difference compared with the control group, as determined by the Tukey-Kramer post test.
Islet transplantation
Sprague-Dawley rats (n=30; male, 6–8 weeks of age, 250–300 g) were rendered diabetic by a single intraperitoneal injection of streptozotocin (STZ, 50 mg/kg). The rats were anesthetized by an intraperitoneal injection of pentobarbital before transplantation. The rats were randomly apportioned into 3 groups as follows: group A, islets (control); group B, SIS-islets; and group C, SIS-MSC-islets. In the controls (group A), the rats received 1,000-islet transplantation under the skin. In group B, 1,000 SIS-coated islets were folded to a size of 1×1 cm and fixed on the back under the skin of the rats. In group C, 5×106 MSCs were seeded onto SIS to generate an SIS-MSC scaffold within 48 h, and 1,000 islets were coated and folded as in group B for transplantation. No immunosuppressive protocols were applied in the recipient rats.
Graft survival and function
To assess islet graft survival and function, blood glucose and insulin concentrations were monitored. Blood was obtained from the tail vein using a syringe. Successful islet function was defined as blood glucose levels <11.1 mmol/l on 2 consecutive days. Graft rejection was defined as blood glucose levels >11.1 mmol/l on 2 consecutive days. The mean survival time of the grafts was recorded.
Statistical analysis
The statistical significance of the differences was determined using one-way analysis of variance. Statistical analyses were performed using SPSS17.0 software. A P-value <0.05 was considered to indicate a statistically significant difference. Differences among the groups with regard to blood glucose and insulin concentration were analyzed by multivariate analysis.
Results
Characterization of MSCs, islets and SIS
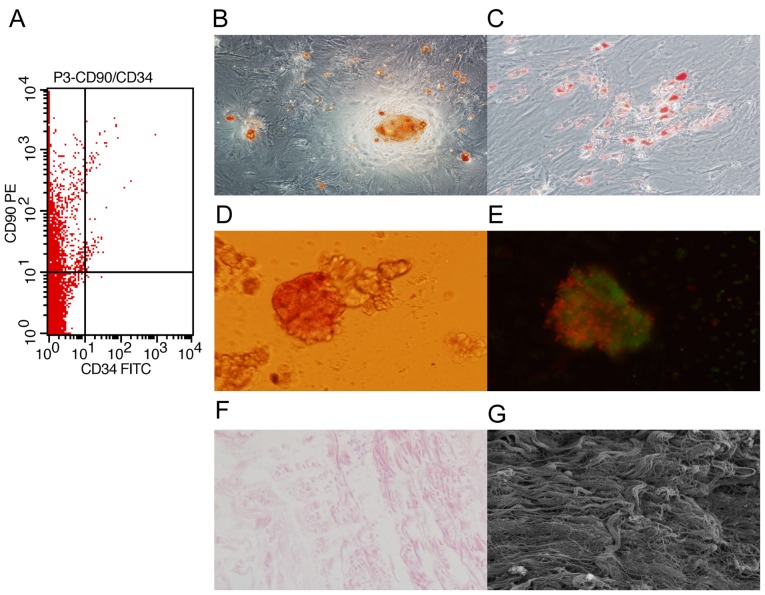
To characterize the MSCs, bone marrow cells isolated from rats were stained for surface molecular marks (CD90 and CD34) and analyzed by flow cytometry. We found that the cells were positive for CD90 and negative for CD34 (Fig. 1A). In addition, the cells were able to differentiate into osteoblast-like and adipocyte-like cells (Fig. 1B and C). These results confirmed that the isolated cells were MSCs.
Figure 1.
Characterization of mesenchymal stem cells (MSCs), islets and small intestinal submucosa (SIS). MSCs and islets were isolated from Sprague-Dawley rats and SIS was prepared from Bamei pigs. (A) Flow cytometric analysis shows the MSC phenotype of the cells (CD90-positive and CD34-negative). MSCs differentiated into (B) osteoblast-like and (C) adipocyte-like cells. Islets were stained with (D) dithizone and (E) acridine orange/propidium iodide (AO/PI). (F) SIS was identified by H&E staining and (G) scanning electron microscopy.
To characterize the islets, islets isolated from rats were identified with dithizone and AO/PI staining. We found that the islets were stained with dithizone and AO/PI (Fig. 1D and E). These results indicated that islet isolation was successful.
To characterize SIS, the SIS from Bamei pigs was observed respectively under a light microscope and scanning electron microscope. We found that the SIS was composed of collagen fiber with no cells (Fig. 1F and G), indicating that the isolation was successful.
SIS-MSC scaffold enhances islet viability and function in vitro
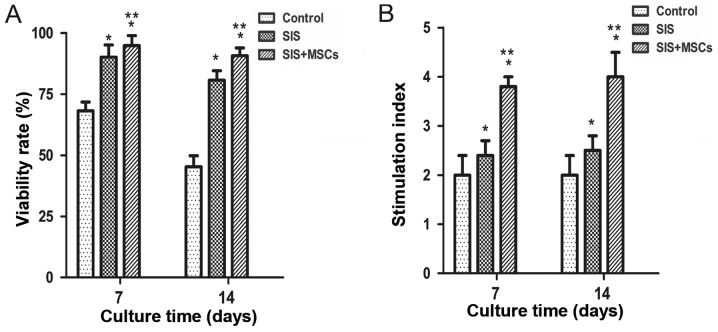
To examine the effects of the SIS and SIS-MSC scaffold on islets, their viability and function were examined in vitro. We found that the viability was significantly higher in both the SIS group and SIS-MSC group than in the control group (Fig. 2A). The cell viability in the SIS-MSC group appeared to be superior to that of the SIS group. These results suggest that the SIS and SIS-MSC scaffold increase islet viability.
Figure 2.
Small intestinal submucosa-mesenchymal stem cell (SIS-MSC) scaffold enhances islet viability and function in vitro. (A) Islet viability and (B) insulin release SI in the control, SIS, and SIS-MSC groups. All samples are presented as the means ± SEM, *P<0.05 compared to the control group; **P<0.05 compared to the SIS group, n=10 cells isolated from 10 rats.
Islet function was determined with a glucose-stimulated insulin secretion test on days 7 and 14. We found that the SI was significantly higher in both the SIS and SIS-MSC groups relative to the control group (Fig. 2B; P<0.05). The SI was significantly higher in the SIS-MSC group compared with the SIS group (P<0.05). These findings suggest that the SIS and SIS-MSC scaffolds enhanced islet function, and that the SIS-MSC scaffold was superior to the SIS scaffold.
SIS-MSC scaffold increases insulin expression in islets in vitro
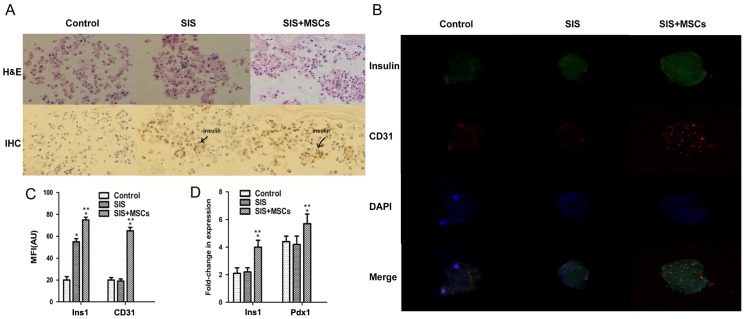
To investigate whether SIS-MSC increases insulin secretion, we analyzed the intensity of insulin staining in the islets by immunohistochemistry and immunofluorescence staining. The results of immunohistochemistry revealed that the intensity of insulin was significantly higher in the SIS-MSC group than in either the SIS group or the control group (Fig. 3A). The insulin signal was undetectable and islet morphology became loose in the control group, whereas the insulin signal was detected and islet morphology was compact in the SIS and SIS-MSC groups. Consistently, the results of immunofluorescence staining indicated that the MFI of insulin was markedly higher in the SIS-MSC group than in the SIS or the control groups (Fig. 3B and C). These results revealed that the SIS-MSC scaffold was associated with an increase in insulin levels and may prevent islet destruction.
Figure 3.
Small intestinal submucosa-mesenchymal stem cell (SIS-MSC) scaffold upregulates insulin and CD31 expression in vitro. (A) Detection of insulin in the control, SIS, and SIS-MSC groups by H&E staining and immunohistochemistry. (B) Double-immunofluorescence staining of insulin and CD31. (C) MFI of insulin and CD31. (D) Insulin 1 (Ins1) and pancreatic and duodenal homeobox 1 (Pdx1) mRNA levels. *P<0.05 compared to the control group; **P<0.05 compared to the SIS group, n=10 cells isolated from 10 rats.
Subsequently, we examined the gene expression levels of Ins1 and Pdx1 by RT-qPCR. We found that the levels of Ins1 and Pdx1 were significantly higher in the SIS-MSC group than in the SIS and the control groups, and that there was no significant difference in mRNA levels of Ins1 or Pdx1 between the control and SIS groups (Fig. 3D). These results suggest that the SIS-MSC scaffold rather than the SIS scaffold upregulates the gene expression of Pdx1 and Ins1.
SIS-MSC scaffold increases CD31 expression in islets in vitro
CD31 is a marker of the vascular endothelium (31). To investigate whether the SIS-MSC scaffold improves the microcirculation of islets, we performed an immunofluorescence analysis for CD31. Although the islets were positive for CD31 in the 3 groups, the MFI of CD31 was significantly higher in the SIS-MSC group than in the SIS and the control group (Fig. 3B–C). These results suggest that SIS-MSC scaffold boosts islet microcirculation.
SIS-MSC scaffold increases growth factor secretion and decreases TNF secretion in vitro
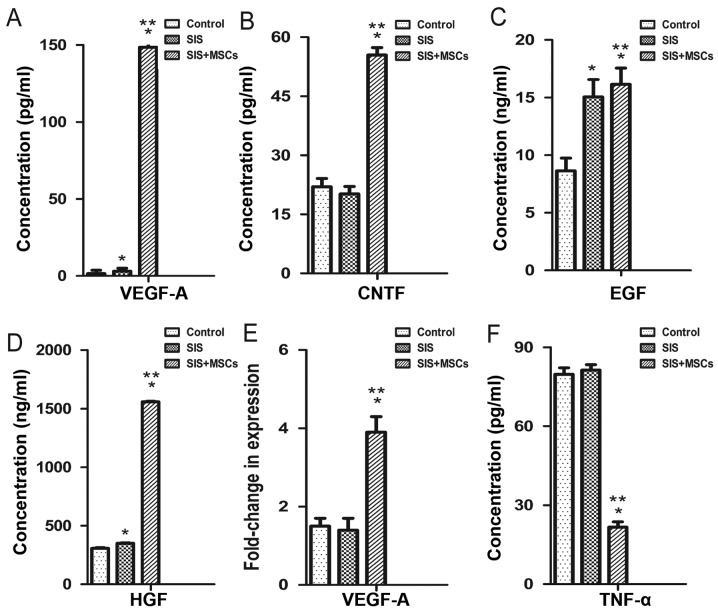
We examined the effects of the SIS-MSC scaffold on cytokine secretion using ELISA. The concentrations of VEGFA, CNTF, EGF and HGF in culture media were significantly higher in the SIS-MSC group than in the SIS group or the control group (Fig. 4A–D). Consistently, the results of RT-qPCR revealed that the mRNA levels of Vegfa were significantly higher in the SIS-MSC group compared with the SIS or the control groups (Fig. 4E). By contrast, the concentrations of TNF in the culture media were significantly lower in the SIS-MSC group than in the SIS or the control groups (Fig. 4F). These results suggest that MSCs can secrete growth factors and may decrease inflammation.
Figure 4.
Small intestinal submucosa-mesenchymal stem cell (SIS-MSC) scaffold increases growth factor secretion and decreases tumor necrosis factor (TNF) secretion in vitro. Effects of SIS-MSC scaffold on cytokine secretion were examined in the control, SIS and SIS-MSC groups. Concentrations of vascular endothelial growth factor A (VEGFA), CNTF, EGF, HGF and TNF in cultured supernatants were examined by ELISA (A–D and F). VEGFA mRNA levels were examined by RT-qPCR in the 3 groups (E). All samples are presented as the means ± SEM, *P<0.05 compared to control group; **P<0.05 compared to the SIS group, n=10 cells isolated from 10 rats.
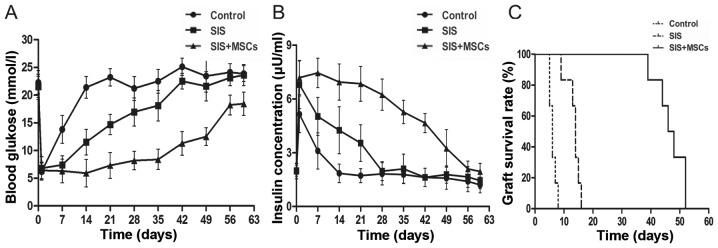
SIS-MSC scaffold improves islet function and graft survival in vivo
To examine the effects of the SIS-MSC scaffold on graft survival and function, we performed islet transplantation in rats and monitored the blood levels of glucose and insulin. While the blood glucose levels were significantly lower in both the SIS and the SIS-MSC groups than in the control group, these levels were markedly lower in the SIS-MSC group than in the SIS group (Fig. 5A). Consistently, the blood insulin levels and graft survival time were significantly higher in the SIS-MSC group relative to the SIS or the control groups (Fig. 5B and C). These findings suggest that the SIS-MSC scaffold improves islet function and prolongs graft survival.
Figure 5.
Small intestinal submucosa-mesenchymal stem cell (SIS-MSC) scaffold improves islet graft function and survival. Islet transplantation was performed in the control, SIS, and SIS-MSC groups. Blood levels of (A) glucose and (B) insulin were monitored, and (C) the survival time the of grafts was recorded. All samples are presented as the means ± SEM, n=10 rats in each group.
Discussion
In this study, we investigated the effects of the SIS-MSC scaffold on islet function and survival. We found that the SIS-MSC scaffold significantly improved islet function and survival in vitro and in vivo.
MSCs have become a promising source for cell-based therapies (32,33). It has been reported that the co-culture of islets with MSCs has beneficial effects, including maintaining morphological changes, conserving islet function and preventing an early inflammatory reaction (34,35). Recently, SIS has been used clinically as a safe material to repair vascular, urogenital and musculoskeletal tissues. SIS is a superior biomaterial due to its biodegradability, biocompatibility, and low rate of peritoneal adhesions (36). In this study, we generated a new scaffold containing both MSCs and SIS and investigated its effect on islets.
In the pancreas, extracellular matrix (ECM) encircles the islets to provide support, mediate adhesion and activate signaling pathways (10). Upon isolation and purification, the loss of ECM and cell-cell interactions leads to rapid islet death (37). Our findings demonstrated that SIS and SIS-MSC scaffolds increased the viability and function of islets. These results suggest that SIS, which has a 3-dimensional structure, may protect the ECM and cell-cell interactions, thus decreasing the loss of islets.
Our study demonstrated that the expression of insulin and Pdx1 was upregulated in islets coated by SIS-MSC. Pdx1 is an important transcription factor that plays an essential role in the development of the pancreas, islet differentiation and the maintenance of β-cell function (38). It may also regulate islet cell proliferation and apoptosis (39). Previous studies have indicated that MSCs are associated with an increase in the expression of some islet-related genes, particularly Pdx1 and insulin (39,40).
Our results revealed that the SIS-MSC scaffold may conserve islet microcirculation and maintain islet morphology. A dense vascular network in islets is essential for efficient insulin secretion and oxygen transfer (41). In islet transplantation, islets are isolated from the remainder of the pancreas. This process destroys the vasculature within the islets (18). Our results revealed that the SIS-MSC scaffold increased CD31 expression, a marker of vascular endothelium.
Our in vivo results revealed that both the SIS and SIS-MSC scaffolds prolonged the survival of grafts following islet transplantation. SIS, as a physical immunobarrier, can protect islets from contact with blood and avoid an instant blood-mediated inflammatory reaction. However, we found that islet function and graft survival were markedly improved in diabetic rats receiving islets coating the SIS-MSC scaffold, compared with rats receiving islets coating the SIS, suggesting that MSCs contribute to the prolongation of islet survival. It has been shown that MSCs secrete cytokines as a nutrition source for islets. VEGFA can promote vascular development, HGF may enhance endogenous β-cell regeneration (40) and EGF can promote metaplastic-ductal formation (42). We found that the concentrations of VEGFA, CNTF, EGF and HGF in the culture media were significantly higher in the SIS-MSC group than in the SIS group. Of note, the concentrations of TNF, which play important pro-inflammatory and pro-apoptotic roles in islet transplantation (43), were lower in the SIS-MSC group, suggesting that MSCs may decrease inflammation.
In conclusion, the findings of our study demonstrated that the SIS-MSC scaffold significantly improved islet function and islet survival in vitro and in vivo. This improvement may be associated with the upregulation of insulin expression, the improvement of islet microcirculation and the secretion of cytokines.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (grant no. 81270548).
References
- 1.Shi Y, Hu FB. The global implications of diabetes and cancer. Lancet. 2014;383:1947–1948. doi: 10.1016/S0140-6736(14)60886-2. [DOI] [PubMed] [Google Scholar]
- 2.Inverardi L, Kenyon NS, Ricordi C. Islet transplantation: Immunological perspectives. Curr Opin Immunol. 2003;15:507–511. doi: 10.1016/S0952-7915(03)00115-8. [DOI] [PubMed] [Google Scholar]
- 3.Olabisi RM. Cell microencapsulation with synthetic polymers. J Biomed Mater Res A. 2015;103:846–859. doi: 10.1002/jbm.a.35205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shim JB, Ankeny RF, Kim H, Nerem RM, Khang G. A study of a three-dimensional PLGA sponge containing natural polymers co-cultured with endothelial and mesenchymal stem cells as a tissue engineering scaffold. Biomed Mater. 2014;9:045015. doi: 10.1088/1748-6041/9/4/045015. [DOI] [PubMed] [Google Scholar]
- 5.O'Sullivan ES, Vegas A, Anderson DG, Weir GC. Islets transplanted in immunoisolation devices: A review of the progress and the challenges that remain. Endocr Rev. 2011;32:827–844. doi: 10.1210/er.2010-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su J, Hu BH, Lowe WL, Jr, Kaufman DB, Messersmith PB. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials. 2010;31:308–314. doi: 10.1016/j.biomaterials.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calafiore R, Basta G, Luca G, Boselli C, Bufalari A, Giustozzi GM, Moggi L, Brunetti P. Alginate/polyaminoacidic coherent microcapsules for pancreatic islet graft immunoisolation in diabetic recipients. Ann NY Acad Sci. 1997;831:313–322. doi: 10.1111/j.1749-6632.1997.tb52206.x. [DOI] [PubMed] [Google Scholar]
- 8.Qi M, Gu Y, Sakata N, Kim D, Shirouzu Y, Yamamoto C, Hiura A, Sumi S, Inoue K. PVA hydrogel sheet macroencapsulation for the bioartificial pancreas. Biomaterials. 2004;25:5885–5892. doi: 10.1016/j.biomaterials.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 9.Lamb M, Storrs R, Li S, Liang O, Laugenour K, Dorian R, Chapman D, Ichii H, Imagawa D, Foster C, III, et al. Function and viability of human islets encapsulated in alginate sheets: In vitro and in vivo culture. Transplant Proc. 2011;43:3265–3266. doi: 10.1016/j.transproceed.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Davis NE, Beenken-Rothkopf LN, Mirsoian A, Kojic N, Kaplan DL, Barron AE, Fontaine MJ. Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel. Biomaterials. 2012;33:6691–6697. doi: 10.1016/j.biomaterials.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qureshi KM, Lee J, Paget MB, Bailey CJ, Curnow SJ, Murray HE, Downing R. Low gravity rotational culture and the integration of immunomodulatory stem cells reduce human islet allo-reactivity. Clin Transplant. 2015;29:90–98. doi: 10.1111/ctr.12488. [DOI] [PubMed] [Google Scholar]
- 12.McPherson TB, Badylak SF. Characterization of fibronectin derived from porcine small intestinal submucosa. Tissue Eng. 1998;4:75–83. doi: 10.1089/ten.1998.4.75. [DOI] [Google Scholar]
- 13.Prevel CD, Eppley BL, Summerlin DJ, Sidner R, Jackson JR, McCarty M, Badylak SF. Small intestinal submucosa: Utilization as a wound dressing in full-thickness rodent wounds. Ann Plast Surg. 1995;35:381–388. doi: 10.1097/00000637-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 14.D'Eredità R. Porcine small intestinal submucosa (SIS) myringoplasty in children: A randomized controlled study. Int J Pediatr Otorhinolaryngol. 2015;79:1085–1089. doi: 10.1016/j.ijporl.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 15.Ferrand BK, Kokini K, Badylak SF, Geddes LA, Hiles MC, Morff RJ. Directional porosity of porcine small-intestinal submucosa. J Biomed Mater Res. 1993;27:1235–1241. doi: 10.1002/jbm.820271004. [DOI] [PubMed] [Google Scholar]
- 16.Badylak S, Liang A, Record R, Tullius R, Hodde J. Endothelial cell adherence to small intestinal submucosa: An acellular bioscaffold. Biomaterials. 1999;20:2257–2263. doi: 10.1016/S0142-9612(99)00156-8. [DOI] [PubMed] [Google Scholar]
- 17.Woods EJ, Walsh CM, Sidner RA, Zieger MA, Mullin S, Lakey JR, Ricordi C, Critser JK. Enhanced recovery of cryopreserved islets using SIS. Transplant Proc. 2004;36:1139–1142. doi: 10.1016/j.transproceed.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Xiaohui T, Wujun X, Xiaoming D, Xinlu P, Yan T, Puxun T, Xinshun F. Small intestinal submucosa improves islet survival and function in vitro culture. Transplant Proc. 2006;38:1552–1558. doi: 10.1016/j.transproceed.2006.02.134. [DOI] [PubMed] [Google Scholar]
- 19.Lakey JRT, Troendle J, Zieger MAJ, Geary WA, Voytek S, Critser JK. Improved islet survival and in vitro function using small intestinal submucosa. Transplant Proc. 1998;30:383–383. doi: 10.1016/S0041-1345(97)01320-1. [DOI] [PubMed] [Google Scholar]
- 20.Zaher W, Harkness L, Jafari A, Kassem M. An update of human mesenchymal stem cell biology and their clinical uses. Arch Toxicol. 2014;88:1069–1082. doi: 10.1007/s00204-014-1232-8. [DOI] [PubMed] [Google Scholar]
- 21.Secunda R, Vennila R, Mohanashankar AM, Rajasundari M, Jeswanth S, Surendran R. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: A comparative study. Cytotechnology. 2015;67:793–807. doi: 10.1007/s10616-014-9718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagamura-Inoue T, Mukai T. Umbilical cord is a rich source of mesenchymal stromal cells for cell therapy. Curr Stem Cell Res Ther. 2015 Oct 26; doi: 10.2174/1574888x10666151026115017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metal-loproteinase-2 and -9. Diabetes. 2009;58:1797–1806. doi: 10.2337/db09-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itakura S, Asari S, Rawson J, Ito T, Todorov I, Liu CP, Sasaki N, Kandeel F, Mullen Y. Mesenchymal stem cells facilitate the induction of mixed hematopoietic chimerism and islet allograft tolerance without GVHD in the rat. Am J Transplant. 2007;7:336–346. doi: 10.1111/j.1600-6143.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 25.Shin JY, Jeong JH, Han J, Bhang SH, Jeong GJ, Haque MR, Al-Hilal TA, Noh M, Byun Y, Kim BS. Transplantation of heterospheroids of islet cells and mesenchymal stem cells for effective angiogenesis and antiapoptosis. Tissue Eng Part A. 2015;21:1024–1035. doi: 10.1089/ten.tea.2014.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshimatsu G, Sakata N, Tsuchiya H, Minowa T, Takemura T, Morita H, Hata T, Fukase M, Aoki T, Ishida M, et al. The co-transplantation of bone marrow derived mesenchymal stem cells reduced inflammation in intramuscular islet transplantation. PLoS One. 2015;10:e0117561. doi: 10.1371/journal.pone.0117561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borg DJ, Weigelt M, Wilhelm C, Gerlach M, Bickle M, Speier S, Bonifacio E, Hommel A. Mesenchymal stromal cells improve transplanted islet survival and islet function in a syngeneic mouse model. Diabetologia. 2014;57:522–531. doi: 10.1007/s00125-013-3109-4. [DOI] [PubMed] [Google Scholar]
- 28.Gao X, Song L, Shen K, Wang H, Qian M, Niu W, Qin X. Bone marrow mesenchymal stem cells promote the repair of islets from diabetic mice through paracrine actions. Mol Cell Endocrinol. 2014;388:41–50. doi: 10.1016/j.mce.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47:74–80. doi: 10.1016/0022-4804(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 30.Rackham CL, Chagastelles PC, Nardi NB, Hauge-Evans AC, Jones PM, King AJ. Co-transplantation of mesenchymal stem cells maintains islet organisation and morphology in mice. Diabetologia. 2011;54:1127–1135. doi: 10.1007/s00125-011-2053-4. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Zhao R, Liu J, Tian M, Lu Y, He T, Cheng M, Liang K, Li X, Wang X, et al. Small islets transplantation superiority to large ones: Implications from islet microcirculation and revascularization. J Diabetes Res. 2014;2014:192093. doi: 10.1155/2014/192093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64:587–592. doi: 10.2337/db14-0656. [DOI] [PubMed] [Google Scholar]
- 33.Franchi F, Peterson KM, Xu R, Miller B, Psaltis PJ, Harris PC, Lerman LO, Rodriguez-Porcel M. Mesenchymal Stromal Cells Improve Renovascular Function in Polycystic Kidney Disease. Cell Transplant. 2015;24:1687–1698. doi: 10.3727/096368914X684619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sumi S, Yanai G. Fusion of mesenchymal stem cells and islet cells for cell therapy. Methods Mol Biol. 2015;1313:107–113. doi: 10.1007/978-1-4939-2703-6_7. [DOI] [PubMed] [Google Scholar]
- 35.Rackham CL, Vargas AE, Hawkes RG, Amisten S, Persaud SJ, Austin ALF, King AJF, Jones PM. Annexin A1 is a key modulator of Mesenchymal Stromal Cell mediated improvements in islet function. Diabetes. 2016;65:129–139. doi: 10.2337/db15-0990. [DOI] [PubMed] [Google Scholar]
- 36.Costa RG, Lontra MB, Scalco P, Cavazzola LT, Gurski RR. Polylactic acid film versus acellular porcine small intestinal submucosa mesh in peritoneal adhesion formation in rats. Acta Cir Bras. 2009;24:128–135. doi: 10.1590/S0102-86502009000200010. [DOI] [PubMed] [Google Scholar]
- 37.Schaschkow A, Mura C, Bietiger W, Peronet C, Langlois A, Bodin F, Dissaux C, Bruant-Rodier C, Pinget M, Jeandidier N, et al. Impact of an autologous oxygenating matrix culture system on rat islet transplantation outcome. Biomaterials. 2015;52:180–188. doi: 10.1016/j.biomaterials.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 38.Lin P, Li W, Yao Z, Sun Y, Wang L, Li S, Chen L. Oral administration of PDX1 confers protection against insulitis in the non-obese diabetic (NOD) mice. Biochem Biophys Res Commun. 2015;466:656–663. doi: 10.1016/j.bbrc.2015.09.098. [DOI] [PubMed] [Google Scholar]
- 39.Yuan H, Liu H, Tian R, Li J, Zhao Z. Regulation of mesenchymal stem cell differentiation and insulin secretion by differential expression of Pdx-1. Mol Biol Rep. 2012;39:7777–7783. doi: 10.1007/s11033-012-1619-7. [DOI] [PubMed] [Google Scholar]
- 40.Boumaza I, Srinivasan S, Witt WT, Feghali-Bostwick C, Dai Y, Garcia-Ocana A, Feili-Hariri M. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun. 2009;32:33–42. doi: 10.1016/j.jaut.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Zanone MM, Favaro E, Camussi G. From endothelial to beta cells: Insights into pancreatic islet microendothelium. Curr Diabetes Rev. 2008;4:1–9. doi: 10.2174/157339908783502415. [DOI] [PubMed] [Google Scholar]
- 42.Lai M, Cai K, Hu Y, Zhang Y, Li L, Luo Z, Hou Y, Li J, Ding X, Chen X. Construction of microenvironment onto titanium substrates to regulate the osteoblastic differentiation of bone marrow stromal cells in vitro and osteogenesis in vivo. J Biomed Mater Res A. 2013;101:653–666. doi: 10.1002/jbm.a.34371. [DOI] [PubMed] [Google Scholar]
- 43.SoRelle JA, Itoh T, Peng H, Kanak MA, Sugimoto K, Matsumoto S, Levy MF, Lawrence MC, Naziruddin B. Withaferin A inhibits pro-inflammatory cytokine-induced damage to islets in culture and following transplantation. Diabetologia. 2013;56:814–824. doi: 10.1007/s00125-012-2813-9. [DOI] [PubMed] [Google Scholar]