Abstract
Fibrosis in skeletal muscle develops after injury or in response to chronic kidney disease (CKD) but the origin of cells becoming fibrous tissue and the initiating and sustaining mechanisms causing muscle fibrosis are unclear. We have identified muscle fibro/adipogenic progenitor cells (FAPs) that potentially differentiate into adipose tissues or fibrosis. We also demonstrated that CKD stimulates myostatin production in muscle. Therefore, we tested whether CKD induces myostatin which stimulates fibrotic differentiation of FAPs leading to fibrosis in skeletal muscles. We isolated FAPs from mouse muscles and found that myostatin stimulates their proliferation and conversion into fibrocytes. In vivo, FAPs isolated from EGFP-transgenic mice (FAPs-EGFP) were transplanted into muscles of mice with CKD or into mouse muscles that were treated with myostatin. CKD or myostatin stimulated FAPs-EGFP proliferation in muscle and increased α-smooth muscle actin expression in FAP-EGFP cells. When myostatin was inhibited with a neutralizing peptibody (a chimeric peptide-Fc fusion protein), the FAP proliferation and muscle fibrosis induced by CKD were both suppressed. Knocking down Smad3 in cultured FAPs interrupted their conversion into fibrocytes indicating that myostatin directly converts FAPs into fibrocytes. Thus, counteracting myostatin may be a strategy for preventing the development of fibrosis in skeletal muscles of patients with CKD.
Keywords: Fibrosis, Chronic kidney disease (CKD), Mesenchymal progenitor cells, Myostatin
Introduction
Diseases that injure the kidney, heart, liver, lung, skin and potentially other organs can initiate a series of complex cellular and molecular events producing fibrosis which leads to cellular dysfunction and ultimately, organ failure 1;2. Despite developments in understanding the pathogenesis of fibrosis in many organs, there are no routinely successful treatments that block the development of fibrosis. The difficulties in designing anti-fibrotic treatments are related in part to problems with identifying the origin of cells that produce the proteins which contribute to the development of fibrosis and uncovering the pathways that lead to fibrosis 2. For example, LeBleu et al., investigated cells that develop into fibrocytes in damaged kidneys and concluded that different cells contribute to the development of fibrosis in the injured kidneys 3. There also is evidence that cells initiating fibrosis in injured kidney or lung tissues arise following epithelial-mesenchymal transition (EMT) 4;5. Others report that kidney fibrosis develops following activation of bone marrow-derived cells or perivascular fibrocytes although the involvement of these cells in elaboration of collagen type 1 is controversial 3;4;6. Responses to injury in other organs such as the liver involve precursors of perivascular mesenchymal cells (e.g., hepatic stellate cells). They contribute to the development of hepatic fibrosis but the involvement of other cells has not been excluded 7. In the generation of cardiac fibrosis, there is evidence that TGF-β1 initiates EMT with increased activity of fibrocytes although fibrosis in the heart also could involve other cells 8.
Mechanisms that initiate fibrosis in skeletal muscles has received minimal attention and is not even discussed in a popular review 2. In investigating muscle fibrosis, we found that changes in IGF-1 in mice with chronic kidney disease (CKD) contributes to fibrosis in injured muscles but we did not identify specific cells that produce fibrosis nor did the pathway that stimulates muscle fibrosis 1. In injured skeletal muscles, one potential precursor of fibrosis is the satellite cell because they possess multilineage capabilities including myogenic, adipogenic and fibrogenic properties 9;10. In fact, impaired functions of satellite cells in mice with CKD or in aging mice is associated with skeletal muscle fibrosis 1;11. Mice bearing satellite cell mutations (e.g., in mice with Pax7 knockout (KO) or with genetic deletions of Myogenin, Myf5 or MyoD) experience accumulation of adipocytes and muscle fibrosis after muscle injury 12–15. However, an evaluation of lineage-tracing in MyoD-Cre/R26R-EYFP mice concluded that satellite cells do not spontaneously develop into adipocytes or fibrocytes 16. Besides satellite cells, we and others have uncovered a mesenchymal progenitor cells also called fibro/adipogenic progenitor cells (FAPs) in muscle that can develop into adipocytes or fibrocytes 17–20. These FAPs express PDGFRα and contribute to adipocyte formation in skeletal muscles of mice that are treated with glucocorticoids (GC) 17. FAPs also can stimulate myogenesis 17;20. Although mice engineered to overexpress PDGFRα develop systemic fibrosis, the pathway stimulating fibrosis following muscle injury has not been identified 21. In these experiments, we used in vitro and in vivo techniques to identify how CKD stimulates the development of fibrosis in skeletal muscles. Our results reveal that CKD-induced fibrosis in skeletal muscles originates from FAPs and blocking myostatin suppresses muscle fibrosis in mice with CKD.
Results
CKD induces fibrosis in skeletal muscle
CKD in male mice was created by subtotal nephrectomy in 2 stages over 2 weeks. Results from CKD mice were compared to those of sham-operated, pair fed mice 22. After recovery from surgery, mice were fed 40% protein yielding BUN values >80 mg/dL vs. ~20 mg/dL in control mice 22;23. After 5 months, Sirius Red staining of tibialis anterior (TA) muscles revealed increased collagen deposition in CKD mice vs. control mice (Figure 1A). In gastrocnemius muscles, CKD led to higher mRNAs of fibrosis markers (Figure 1B). Thus, CKD stimulates fibrosis in muscles.
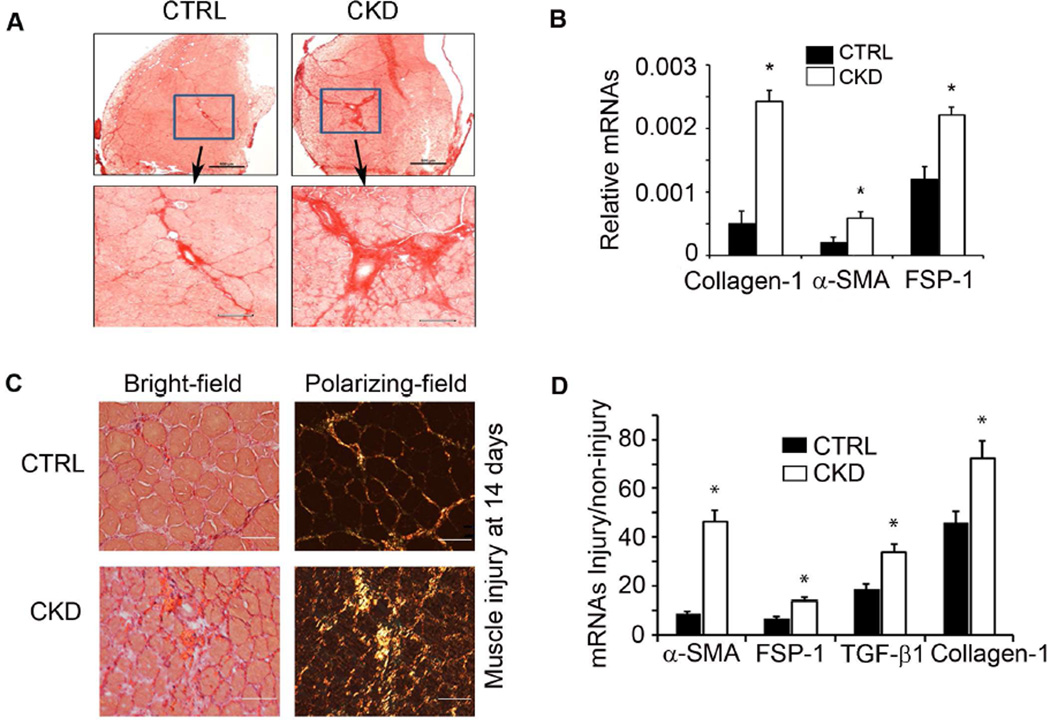
Figure 1. CKD increases muscle fibrosis.
A. Cryo-cross sections of TA muscles were subjected to Sirius Red stainning (bar=50µm); CKD was associated with increased collagen deposition. B. Fibrotic marker mRNAs measured by RT-PCR were higher in gastrocnemius muscles of mice with CKD (n=4; *, P<0.05 vs. control). C. TA muscles were injured by cardiotoxin injection and compared to the contralateral muscle injected with PBS. After 14 days, Sirius red stained cryo-cross-sections of muscles of CKD mice (bar=50µm) revealed more fibrous tissue. D. mRNAs of markers of fibrosis genes were increased in injured muscles of CKD mice (n=4; *, P<0.05 vs. control).
To evaluate whether CKD also stimulates muscle fibrosis in a model of muscle injury, we injected cardiotoxin into mouse TA muscles, a standard model of muscle injury 24. After 14 days of muscle injury, mice with CKD exhibited a significant increase (P<0.05) in collagen deposition (Figure 1C). Muscle injury in mice with CKD also had a significant increase in the mRNAs of fibrotic genes (Figure 1D). Thus, CKD in mice increases muscle fibrosis in both uninjured (Figure 1A,B) and injured muscles (Figure 1C,D).
Myostatin converts FAPs into fibrocytes
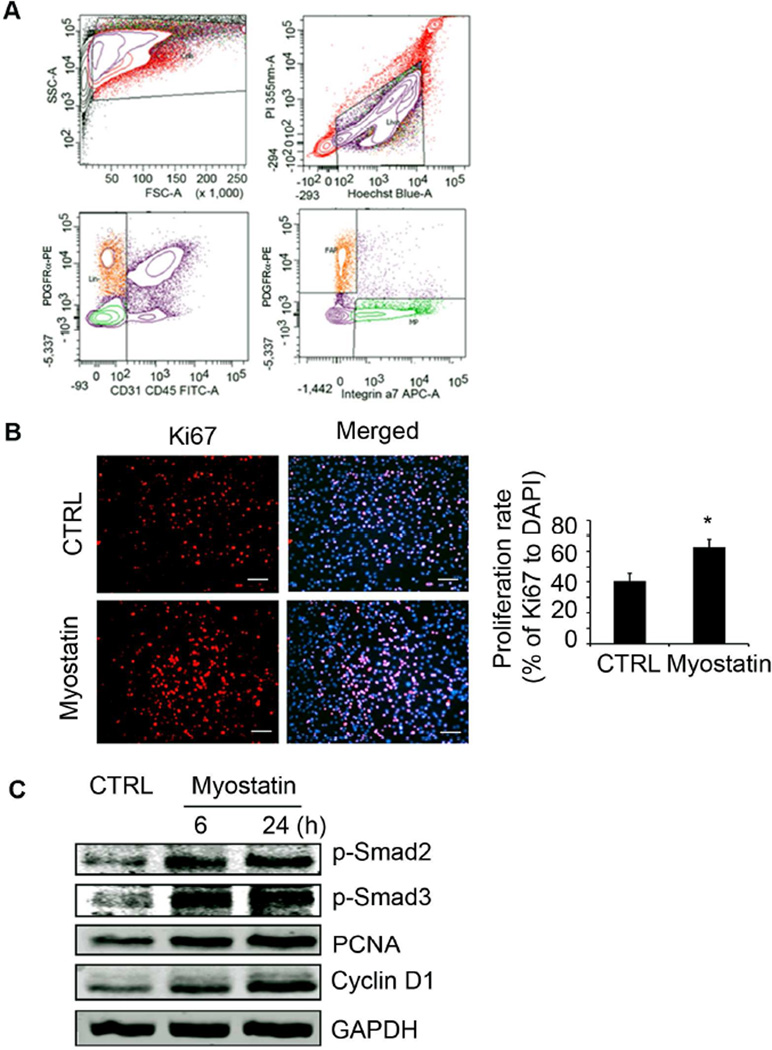
We confirmed our finding that myostatin expression is increased in muscles of mice with CKD (Supplemental Figure S1 A,B) 22. We hypothesized that the increase in myostatin production could stimulate muscle fibrosis and examined isolated FAPs to determine if they can be converted into adipocytes or fibrocytes17–19. FAPs were isolated from muscles of mice using FACS (Fluorescence-activated Cell Sorting) as described (Figure 2A) 17;25. We treated FAPs with 100 nM recombinant myostatin and found there was an increase in their proliferation rate plus increased expression of cyclin D1 and the proliferating cell nuclear antigen (PCNA) (Figure 2 B,C). Thus, myostatin stimulates FAP proliferation in vitro, associated with increases in p-Smad2 and p-Smad3 proteins (Figure 2C).
Figure 2. Myostatin induces FAP proliferation.
A. FACS-isolated FAPs were positive for PDGFRα but negative for CD31, CD45 and integrinα7 (orange cell area). B. FAPs treated with myostatin for 24h were immunostained with anti-Ki67 and its proliferation index is increased (n=3; *, P<0.05 vs. no myostatin). C. FAPs treated with myostatin for 6 or 24h had increased levels of p-Smad2/3 and PCNA and Cyclin D1 (n=3 repeats).
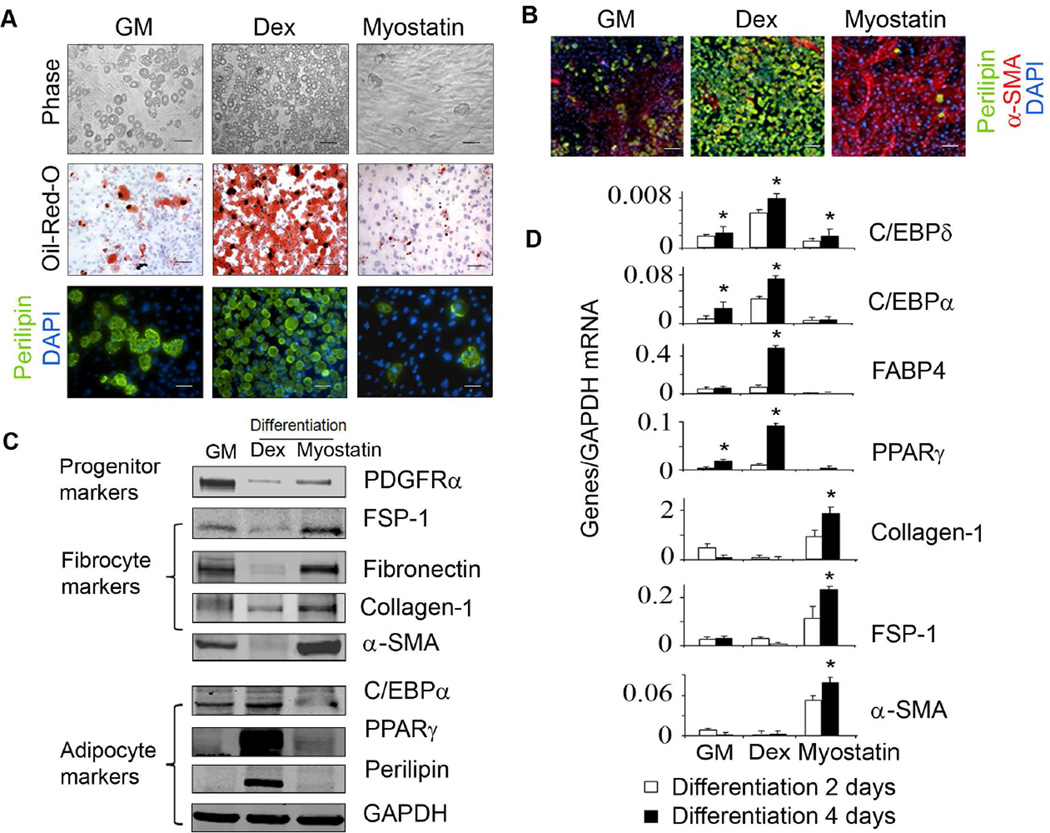
Previously, we found that FAPs were capable of differentiating into adipocytes when they were cultured in an adipogenic differentiation media containing dexmethasone (Dex) 17. Since Dex stimulates myostatin expression in muscle, we examined whether myostatin, like Dex could raise adipogenesis from FAPs 26;27. We added 40 nM myostatin to the adipogenic differentiation media which lacked Dex, there was suppression of adipocyte formation from FAPs vs. results obtained in media containing Dex (Figure 3A). Instead, the added myostatin induced most of the FAPs to develop into α-SMA-positive cells, characteristic of fibrosis (Figure 3B). The protein expression pattern of adipogenic and fibrotic genes were consistent with the immunostaining results: four days after FAPs were exposed to adipogenic media plus Dex, the proteins of adipogenic genes in FAPs were increased. But, when FAPs were cultured in media plus myostatin instead of Dex, protein levels of fibrotic genes were increased (Figure 3C). Differentiation of FAPs into adipocytes or fibrocytes led to a decrease in the expression of the mesenchymal progenitor marker, PDGFRα (Figure 3C). The mRNAs of adipogenic and fibrotic markers were also consistent with the changes in expressed proteins following 2 to 4 days treatment (Figure 3D). We conclude that myostatin stimulates fibrotic differentiation of FAPs resulting in increases in markers of fibrosis.
Figure 3. Myostatin induces fibrotic differentiation of FAPs.
A. After 5 days of incubating FAPs in growth media, cells were switched to adipogenic differentiation media plus Dex or myostatin for 5 days (top panel), or 7 days stained with Oil-RedO (middle panel) or 14 days immunostained with anti-perilipin (bottom panel). B. After 4 days differentiation, FAPs were co-immunostained with anti-α-SMA (red) and anti-perilipin (green). C. FAPs cultured with adipogenic differentiation media plus Dex or myostatin for 4 days were lysed and subjected to western blotting. D. FAPs cultured in adipogenic differentiation media plus Dex or myostatin for 2 or 4 days, mRNAs were evaluated by RT-PCR. (n=3 repeats; *, P<0.05 vs. growth media). In FAPs, Dex increased adipogenic genes while myostatin increased fibrotic genes.
In mice with CKD, muscle fibrosis develops from FAPs
To identify whether the muscle fibrosis found in CKD arises from FAPs, we examined an acute stimulus of fibrosis, muscle injury. Firstly, the number of FAPs (P<0.05) in injured muscles of mice with CKD was greater than results in injured muscles of control mice. There also is a slight increase in FAPs in uninjured muscle of CKD (Figure 4A). To examine whether this increase in FAPs was due to FAP proliferation, we co-immunostained sections of injured TA muscles with antibodies against Ki67 and the FAP marker, PDGFRα. In injured muscles of mice with CKD, the doubly labelled cells were significantly increased (P<0.05), indicating that CKD stimulates the proliferation of FAPs in injured muscles (Figure 4B).
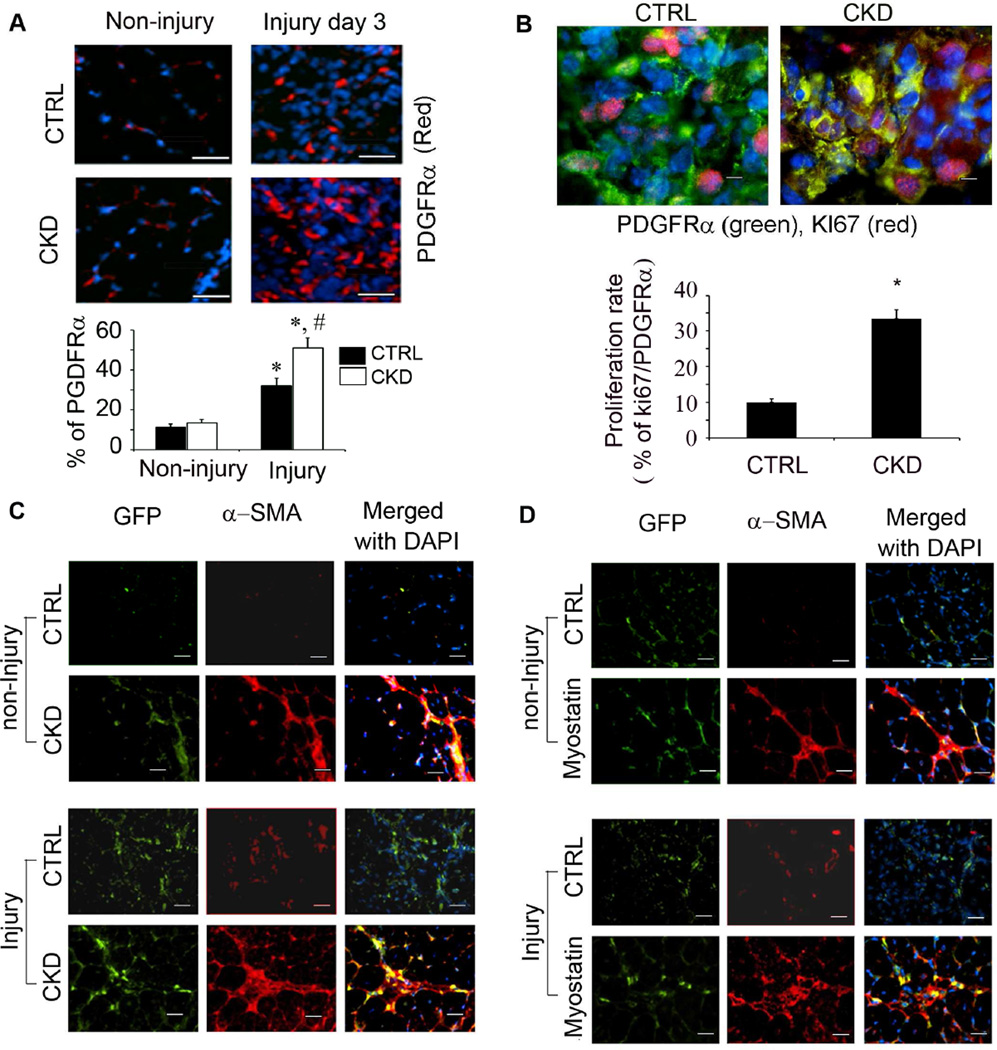
Figure 4. Fibrotic differentiation of FAPs in muscles of mice with CKD is mediated by myostatin.
A. 3 days after muscle injury, sections were immunostained with anti-PDGFRα (red) and the percentage of PDGFRα+ to total nuclear cells (DAPI) were counted (*, P<0.05 vs. control; #, P<0.05 vs. non-injury). CKD increased FAPs in muscle of CKD mice. B. Sections of injured muscles were co-immunostainned with anti- Ki67 and anti-PDGFRα. FAPs proliferation index is higher in injured muscles of CKD mice. C. FAPs-EGFP transplanted into TA muscles (with or without injury) of mice (with or without injury). After 7 days muscle injury and transplantation, sections of muscles were immunostained with anti-α-SMA. CKD or muscle injury increase muscle fibrosis. D. TA muscles were transplanted with FAPs-EGFP and simultaneously treated with myostatin. Sections immunostained with anti-α-SMA (Bar=50 µm) revealed that myostatin stimulated FAP conversion into fibrocytes.
To determine if CKD also induces FAPs to differentiate into fibrosis in vivo, we isolated FAPs from transgenic mice that ubiquitously express EGFP. These FAPs were transplanted into TA muscles of 4 groups of mice to determine if transplanted FAPs-EGFP can be stimulated to express α-SMA: 1) control mice (CTRL); 2) control mice with injured TA muscles; 3) mice with CKD; and 4) mice with CKD and injured TA muscles. The transplanted FAPs did express α-SMA in mice of groups 2, 3 and 4; the largest increase in α-SMA expression was in mice with muscle injury and CKD (group 4; Figure 4C). CKD or injury also stimulated FAP cell proliferation since we found more EGFP+ cells in muscles of mice in group 2, 3 or 4 with vs. control mice even though the same number of FAPs was transplanted into muscles of mice.
To determine if myostatin can mediate the differentiation of FAPs into fibrosis, we transplanted freshly isolated FAPs from EGFP mice into TA muscles. This was followed by an injection of myostatin-soaked beads into the same areas of TA muscles. With this technique, we confined the influence of myostatin from the beads to the same area of muscle bearing FAPs transplanted from EGFP mice. Ten days later, injection of beads bearing myostatin into muscles transplanted with FAPs increased the expression of a-SMA in FAPs that were labelled with EGFP. This pattern of stimulating a-SMA occurred in non-injured and injured muscles of mice that were injected with myostatin (Figure 4D). We conclude that myostatin is stimulated by CKD to activate both the proliferation and differentiation of FAPs into fibrocytes in muscle.
CKD stimulates myostatin to induce FAP differentiation into fibrous tissue
To examine if myostatin mediates muscle fibrosis in mice with CKD, we blocked responses to myostatin by injecting mice with a neutralizing, anti-myostatin peptibody every other day 22. After 5 days, TA muscles were injured by injecting cardiotoxin while the peptibody treatment was continued. Four groups of mice were examined: 1) control mice (CTRL) treated with PBS; 2) control mice treated with the peptibody; 3) CKD mice were treated with PBS; and 4) CKD mice were treated with the peptibody. We also injured right TA muscle of 4 groups of mice to speed up the fibrosis.
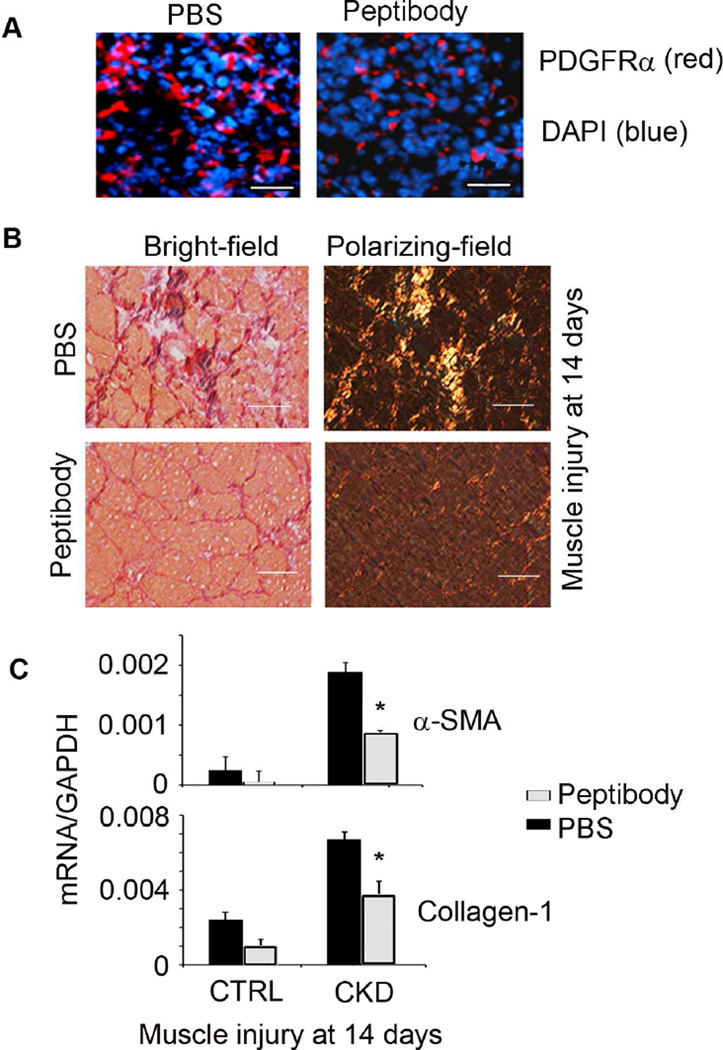
After 3 days of TA muscle injury in CKD mice treated peptibody (Group 4), there was a significant (P<0.05) decrease in the number of FAPs vs. CKD mice treated with PBS (Group 3; Figure 5A). After 14 days of muscle injury, peptibody treatment sharply reduced Sirius Red staining of muscles (Group 4 vs. Group 3 mice) (Figure 5B). Treatment also decreased the mRNAs of α-SMA and collagen-1 ~50% (Figure 5C; P<0.05, group 4 vs. group 3). In Group 2, control mice, myostatin inhibition with the peptibody reduced mRNA levels of fibrosis markers but the differences were not statistically different from results in Group 1) (Figure 5C). The treatment also promoted regeneration of muscles in mice with CKD (Supplemental Figure S2). We conclude that myostatin expression is necessary to stimulate CKD-induced muscle fibrosis.
Figure 5. CKD induces fibrotic differentiation of FAPs via myostatin.
A. Mice with CKD were treated with the anti-myostatin peptibody for 2 weeks. At 3 days after muscle injury, cryo-cross-sections of TA muscles were immunostained with anti-PDGFRα (Bar=50 µm). Myostatin inhibition decreased the number of PDGFRα+ cells. B. At 14 days after muscle injury, peptibody treatment reduced muscle fibrosis. C. Peptibody treatment suppressed the mRNAs of fibrosis markers in muscles of CKD mice.
Myostatin stimulates fibrotic differentiation of FAPs directly
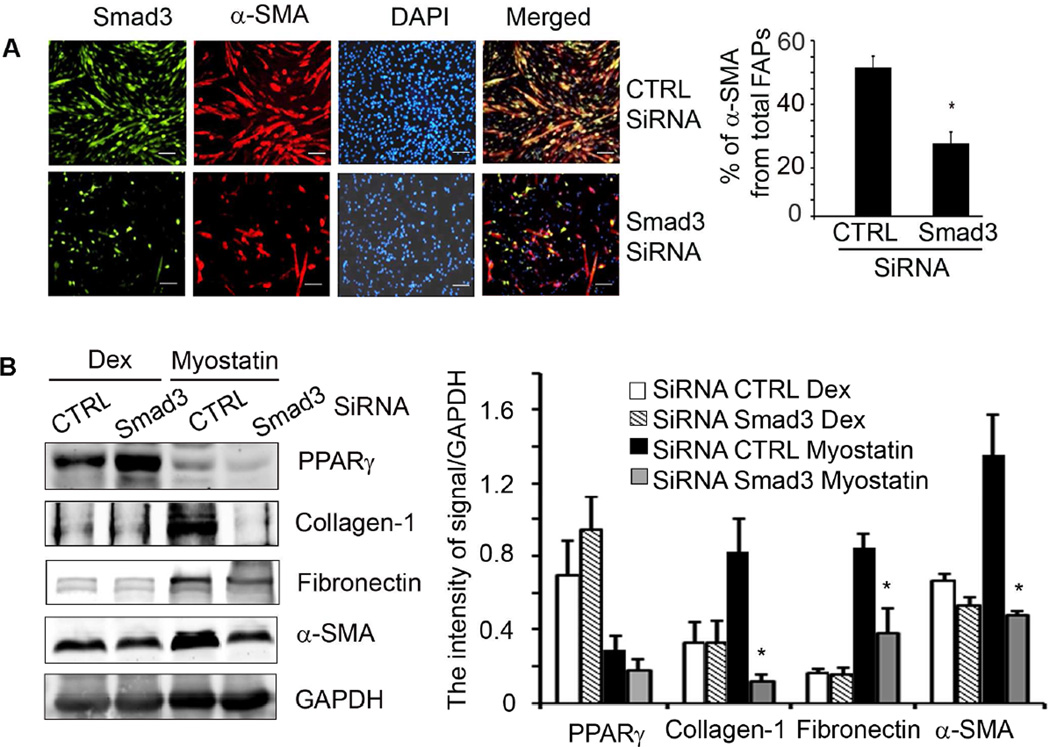
To examine if myostatin directly stimulates differentiation of FAPs into fibrous tissue, in cultured FAPs, we knocked down Smad3 by transfecting FAPs with a lentivirus that expresses Smad3 SiRNA; lentivirus expressing scrambled SiRNA served as a control. After 48 h, the FAPs were treated with myostatin and cells were co-immunostained with anti-Smad3 and α-SMA. Knock down Smad3 blocked myostatin-induced α-SMA expression in FAPs (Figure 6A). We also found that knock down Smad3 does not interfere with the expression of PPARγ indicating that Smad3 is not responsible for adipogenic differentiation of FAPs (Figure 6B). Smad3 knock down did, however, suppress myostatin-induced expression fibrotic markers indicating that myostatin directly stimulates FAPs to differentiate into fibrocytes via a Smad3 signaling pathway (Figure 6B).
Figure 6. Myostatin induces fibrotic differentiation of FAPs via Smad3.
A. Knockdown Smad3 in FAPs decreased myostatin-induced α-SMA cells (Bar=50 µm, *, P<0.05 vs SiRNA CTRL). B. Smad3- knocked down-FAPs cultured in media containing Dex or myostatin for 48h and reavealed that Smad3 knockdown in FAPs suppressed myostatin-induced fibrosis but not Dex-induced adipogenesis. (n=4 repeat; *, P<0.05 vs SiRNA of cells treated with myostatin).
Discussion
Fibrosis in skeletal muscles develops in several chronic illnesses including CKD and muscular dystrophies 2;28;29. In these disorders, myofibers are replaced by extracellular matrix, impairing skeletal muscle functions and counteracting cell and gene therapies for muscle disorders 28. Unfortunately, there are no routinely inhibitory treatments that reverse muscle fibrosis, in part, because the identities of cells producing fibrosis are controversial and pathways initiating muscle fibrosis are poorly defined. Using three strategies, we have investigated the process by which fibrotic tissue develops in skeletal muscle: we studied mice with CKD-induced muscle fibrosis; we investigated whether mesenchymal progenitor cells (FAPs) in muscle transform into fibrocytes; and we evaluated whether myostatin signaling stimulates FAP differentiation into muscle fibrosis. We found that CKD stimulates myostatin production and converts FAPs into muscle fibrocytes via a Smad3 pathway. Thus, we have identified how CKD stimulates fibrosis in muscle.
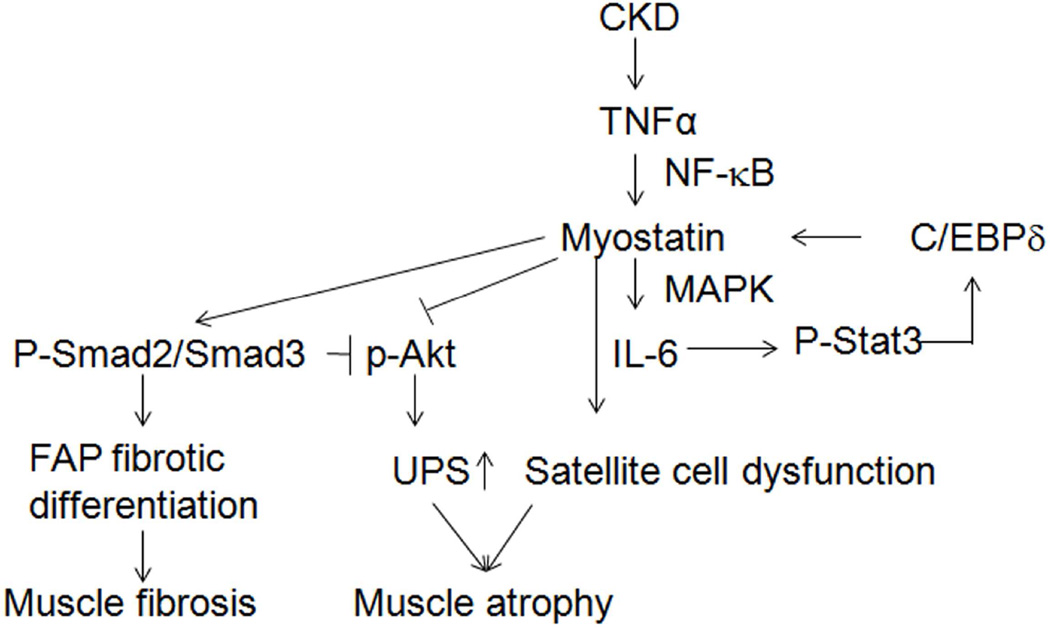
In CKD patients, myostatin production in muscle is inversely proportional to the patient’s eGFR 30;31. In mice with CKD, myostatin levels are increased and linked to the development to muscle atrophy which is suppressed by inhibiting myostatin 22. How does CKD stimulate myostatin production? One possibility is that CKD activates inflammation, signified by increases in circulating cytokines; when we inhibited myostatin in mice with CKD, muscle cytokine expression was suppressed 1;22. Another link between inflammation and myostatin is that mice fed a high-fat diet have increases in circulating TNF-α and IL-6 levels which are reduced in myostatin KO mice 32. Moreover, there is a NF-κB consensus sequence in the myostatin promoter and its activation raises myostatin expression 22;33. We and others find that myostatin stimulates muscle production of IL-6 via MEK and p38 MAPK resulting in impaired satellite cell activity, reduced Akt phosphorylation and causing muscle atrophy 1;22;34. Finally, we find that in CKD, Stat3 activation stimulating myostatin expression through the transcription factor, C/EBPδ 23. Myostatin-induced, catabolic pathways in muscles of mice with CKD are summarized in Figure 7.
Figure 7. Mechanisms initiated by CKD cause atrophy and fibrosis in muscle.
CKD-induced TNF-α stimulates myostatin production via NF-κB 22. Myostatin production is augmented by Stat3 activation which stimulates the transcription factor, C/EBPδ 23. The increase in myostatin impairs satellite cell function and contributes to the loss of muscle mass that is stimulated by CKD 1;22. Myostatin raises Smad2/3 phosphorylation suppressing Akt phosphorylation resulting in activation of the ubiquitin-proteasome system (UPS) and muscle atrophy 22;34. Finally, CKD causes muscle fibrosis because myostatin can convert FAPs into fibrocytes.
The origins of cells developing into muscle fibrosis are controversial. Primary myoblasts or C2C12-derived myoblasts can become muscle fibroblasts 35;36. Other cells developing into fibroblasts include resident or circulating bone marrow 29. We find that FAPs are not only major precursors of muscle fibrocytes but also have multipotential properties including the ability to stimulate satellite cells or their conversion into osteoblasts or adipocytes 17;20. We find (Supplemental figure S3 A) that CKD also increases (P<0.05) the mRNAs of adipocyte genes, PPARγ and C/EBPα in muscle suggesting that CKD-induces adipogenesis from FAPs. This is possible because the increase in glucocorticoids in CKD or diabetes is known to stimulate conversion of FAPs into adipocytes 17;37;38. We speculate that patients with diabetic CKD have increased ectopic accumulation of adipose tissue causing insulin resistance 17.
Besides myostatin, other factors may also affect FAP differentiation. For example, we found that loss of one IGF-1 receptor allele in mice responding to muscle injury actually increases muscle fibrosis 1. In mice with CKD and intact IGF-1 receptor genes, similar results were obtained (Supplemental Figure S3 A,B) raising the possibility that impaired IGF-1 signaling is a mediator of both fibrosis and adipocyte infiltration in muscles of mice with CKD. These results suggest future studies examining whether there is a synergetic relationship between myostatin and IGF-1 signaling that regulates FAP differentiation. There also are interest and contraversal results in the expression of Gdf11 as a means of reversing age-related cardiac hypertrophy with improvement in muscle function 39–42. The role of this factor in CKD is uncertain, because muscle-specific deletion of the Gdf11 gene in mice with Mstn+/+ or Mstn−/− backgrounds, did not increase muscle mass, fiber number, or fiber type 43.
What novel findings have we uncovered? Firstly, CKD can stimulate FAP conversion into fibrocytes (Figure 1). Secondly, myostatin mediates the development of muscle fibrosis (Figure 2–4) 22. Thirdly, myostatin inhibition suppresses muscle fibrosis and increases muscle mass in mice with CKD (Supplemental Figures 2, Figure 5) 22. Fourthly, myostatin directly stimulates the conversion of FAPs into fibroblasts via Smad3 signaling but it does not stimulate the transformation of FAPs into adipocytes (Figure 6) 44. Our results suggest that inhibition of myostatin may be a therapeutic strategy for depressing the development of muscle fibrosis in patients with CKD or chronic, progressive myopathies. Our results may be clinically relevant because Uezumi et al., noted that PDGFRα is present in human muscles while Arrighi et al., concluded that functional properties in human muscles and adipocytes are indistinguishable from events in mice 45;46.
Concise Methods
Animals
Animal experimental procedures were approved by the Baylor College of Medicine IACUC Committee. Anesthetized, C57/BL6 male mice, 8 to 10 week old underwent subtotal nephrectomy in two stages 22; mice with a BUN >80 mg/dl were studied. Sham-operated control mice were pair fed with CKD mice so control mice ate the same amount of food as the CKD mouse on the prior day. Tibialis anterior (TA) muscles were injured by a standard technique of injecting cardiotoxin (Sigma-Aldrich, St. Louis, MO); contralateral muscles were injected with PBS 1;24. To inhibit myostatin, CKD and control mice were subcutaneously injected with an anti-myostatin peptibody (5 mg/kg) every other day 22. This neutralizing, anti-myostatin peptibody suppresses myostatin rather than activin A activity and has an IC50 ~ 1.2 nM (Atara Biopharmaceuticals, Westlake Village, CA).
FAP transplantation
FAPs (PDGFRα positive cells negative for CD31, CD45 and integrin-α7) were isolated using FACS and the Baylor Cell Sorting Core Facility 17;25. Freshly isolated FAPs (5×104) were resuspended in 30 µl Matrigel (BD Bioscience, San Jose, CA) and transplanted into TA muscles of mice with CKD or mice muscle treated with myostatin. To confine myostatin to a specific area in muscle, heparin-agarose beads (125–250 µm diameter; Sigma Aldrich) were incubated in 100 µg/ml myostatin for 1 h at RT. Myostatin-soaked beads were mixed with freshly isolated FAPs and injected into TA muscles. After 10 days, muscles were removed from euthanized mice and used for histological analysis or frozen in liquid nitrogen and stored at −80°C until proteins or RNAs were evaluated.
Cell culture
Isolated FAPs were plated in Matrigel-coated (BD Bioscience) tissue culture plates and cultured in growth media (GM: high-glucose DMEM-supplemented media with 2.5 ng ml−1 bFGF (Invitrogen), 20% FBS and 10% heat-inactivated horse serum). Growth media was then switched to adipogenic differentiation media (DMEM with 10% FBS, 11.5 µg/ml isobutylmethylxanthine (IBMX) and 1 µg/ml insulin) plus 1 µM dexamethasone (Dex) or 40 nM recombinant myostatin. The proliferation index was calculated as Ki67-positive cells divided by the number of cells in a defined area. Investigators counting Ki67-positive cells were masked as to treatments.
Histology and imaging
PBS was perfused through the left ventricle of anesthetized mice and tissues were removed and processed for cryo-sectioning followed by staining with H&E or Sirius Red. Immunostaining was performed using antibodies against perilipin, PDGFRα, α-SMA, GFP or Ki67 as described 23.
RT-PCR analysis
RT-PCR was performed as described with relative gene expression calculated from cycle threshold (Ct) values using GAPDH or 18S as an internal control (relative expression = 2(sample Ct − GAPDH Ct)) 47. Primers sequences are provided upon request.
Statistical Analysis
Results are expressed as means ±SEM. Significance testing was performed using one-way ANOVA followed by pair-wise comparisons using the Student-Newman-Keuls test. Statistical significance was set at P<0.05. A minimum of three replicates were performed for each experimental condition.
Supplementary Material
Acknowledgments
Funding: supported by the National Institutes of Health (R37 DK37175 to W.E.M.; Pilot/Feasibility Award P30-DK079638 to L.Z.); American Diabetic Association (1-11-BS-194 to L. Z.); The National Natural Science Foundation of China (81401780) and Beijing Municipal Natural Science Foundation (5152007) to Y.J.D. Norman S. Coplon extramural research grant and support from Dr. and Mrs. Harold Selzman to L.Z. The experiments were supported by the Baylor Cytometry and Cell Sorting Core, Joel Sederstrom Director, with NIH funds (P30AI036211, P30CA125123, and S10RR024574). Atara Biotherapeutics provided the neutralizing anti-myostatin peptibody.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
Reference List
- 1.Zhang L, Wang XH, Wang H, et al. Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J Am Soc Nephrol. 2010;21:419–427. doi: 10.1681/ASN.2009060571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockey DC, Bell PD, Hill JA. Fibrosis--A Common Pathway to Organ Injury and Failure. N Engl J Med. 2015;373:96. doi: 10.1056/NEJMc1504848. [DOI] [PubMed] [Google Scholar]
- 3.LeBleu VS, Taduri G, O'Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwano M, Plieth D, Danoff TM, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin SL, Kisseleva T, Brenner DA, et al. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 8.Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 9.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 10.Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci. 2004;117:5393–5404. doi: 10.1242/jcs.01419. [DOI] [PubMed] [Google Scholar]
- 11.Brack AS, Conboy MJ, Roy S, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Sci. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 12.Kuang S, Charge SB, Seale P, et al. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun T, Rudnicki MA, Arnold HH, et al. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell. 1992;71:369–382. doi: 10.1016/0092-8674(92)90507-9. [DOI] [PubMed] [Google Scholar]
- 14.Hasty P, Bradley A, Morris JH, et al. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- 15.Rudnicki MA, Schnegelsberg PN, Stead RH, et al. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- 16.Starkey JD, Yamamoto M, Yamamoto S, et al. Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. J Histochem Cytochem. 2011;59:33–46. doi: 10.1369/jhc.2010.956995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong Y, Silva KA, Dong Y, et al. Glucocorticoids increase adipocytes in muscle by affecting IL-4 regulated FAP activity. FASEB J. 2014;28:4123–4132. doi: 10.1096/fj.14-254011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uezumi A, Fukada S, Yamamoto N, et al. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 19.Uezumi A, Ito T, Morikawa D, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 20.Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson LE, Soriano P. Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell. 2009;16:303–313. doi: 10.1016/j.devcel.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Rajan V, Lin E, et al. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J. 2011;25:1653–1663. doi: 10.1096/fj.10-176917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Pan J, Dong Y, et al. Stat3 Activation Links a C/EBPdelta to Myostatin Pathway to Stimulate Loss of Muscle Mass. Cell Metab. 2013;18:368–379. doi: 10.1016/j.cmet.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Ran L, Garcia GE, et al. Chemokine CXCL16 regulates neutrophil and macrophage infiltration into injured muscle, promoting muscle regeneration. Am J Pathol. 2009;175:2518–2527. doi: 10.2353/ajpath.2009.090275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi L, Rossi F. Purification of progenitors from skeletal muscle. J Vis Exp. 2011:e2497. doi: 10.3791/2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma K, Mallidis C, Bhasin S, et al. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am J Physiol. 2003;285:E363–E371. doi: 10.1152/ajpendo.00487.2002. [DOI] [PubMed] [Google Scholar]
- 27.Dong Y, Pan JS, Zhang L. Myostatin suppression of Akirin1 mediates glucocorticoid-induced satellite cell dysfunction. PloS One. 2013;8:e58554. doi: 10.1371/journal.pone.0058554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyer AL, Wagner KR. Regeneration versus fibrosis in skeletal muscle. Curr Opin Rheumatol. 2011;23:568–573. doi: 10.1097/BOR.0b013e32834bac92. [DOI] [PubMed] [Google Scholar]
- 29.Mann CJ, Perdiguero E, Kharraz Y, et al. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verzola D, Procopio V, Sofia A, et al. Apoptosis and myostatin mRNA are upregulated in the skeletal muscle of patients with chronic kidney disease. Kidney Int. 2011;79:773–782. doi: 10.1038/ki.2010.494. [DOI] [PubMed] [Google Scholar]
- 31.Yano S, Nagai A, Isomura M, et al. Relationship between Blood Myostatin Levels and Kidney Function:Shimane CoHRE Study. PLoS One. 2015;10:e0141035. doi: 10.1371/journal.pone.0141035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkes JJ, Lloyd DJ, Gekakis N. Loss-of-function mutation in myostatin reduces tumor necrosis factor alpha production and protects liver against obesity-induced insulin resistance. Diabetes. 2009;58:1133–1143. doi: 10.2337/db08-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma K, Mallidis C, Artaza J, et al. Characterization of 5'-regulatory region of human myostatin gene: regulation by dexamethasone in vitro. Am J Physiol Endocrinol Metab. 2001;281:E1128–E1136. doi: 10.1152/ajpendo.2001.281.6.E1128. [DOI] [PubMed] [Google Scholar]
- 34.Dong Y, Lakhia R, Thomas SS, et al. Interactions between p-Akt and Smad3 in injured muscles initiate myogenesis or fibrogenesis. Am J Physiol Endocrinol Metab. 2013;305:E367–E375. doi: 10.1152/ajpendo.00644.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol. 2007;293:C661–C669. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Foster W, Deasy BM, et al. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164:1007–1019. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.May RC, Bailey JL, Mitch WE, et al. Glucocorticoids and acidosis stimulate protein and amino acid catabolism in vivo. Kidney Int. 1996;49:679–683. doi: 10.1038/ki.1996.96. [DOI] [PubMed] [Google Scholar]
- 38.May RC, Kelly RA, Mitch WE. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J Clin Invest. 1986;77:614–621. doi: 10.1172/JCI112344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loffredo FS, Steinhauser ML, Jay SM, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinha M, Jang YC, Oh J, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Sci. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith SC, Zhang X, Zhang X, et al. GDF11 does not rescue aging-related pathological hypertrophy. Circ Res. 2015;117:926–932. doi: 10.1161/CIRCRESAHA.115.307527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egerman MA, Cadena SM, Gilbert JA, et al. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab. 2015;22:164–174. doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McPherron AC, Huynh TV, Lee SJ. Redundancy of myostatin and growth/differentiation factor 11 function. BMC Dev Biol. 2009;9:24. doi: 10.1186/1471-213X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldman BJ, Streeper RS, Farese RV, Jr, et al. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci U S A. 2006;103:15675–15680. doi: 10.1073/pnas.0607501103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uezumi A, Fukada S, Yamamoto N, et al. Identification and characterization of PDGFRalpha+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014;5:e1186. doi: 10.1038/cddis.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arrighi N, Moratal C, Clement N, et al. Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis. 2015;6:e1733. doi: 10.1038/cddis.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Du J, Hu Z, et al. I-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol. 2009;20:604–612. doi: 10.1681/ASN.2008060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.