Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Serum FLC analysis is a more sensitive indicator of disease than urinalysis.
Improved sensitivity of serum over urine measurements during monitoring translates into valuable prognostic information.
Abstract
Guidelines for monitoring multiple myeloma (MM) patients expressing light chains only (light-chain MM [LCMM]) rely on measurements of monoclonal protein in urine. Alternatively, serum free light chain (sFLC) measurements have better sensitivity over urine methods, however, demonstration that improved sensitivity provides any clinical benefit is lacking. Here, we compared performance of serum and urine measurements in 113 (72κ, 41λ) newly diagnosed LCMM patients enrolled in the Intergroupe Francophone du Myélome (IFM) 2009 trial. All diagnostic samples (100%) had an abnormal κ:λ sFLC ratio, and involved (monoclonal) FLC (iFLC) expressed at levels deemed measurable for monitoring (≥100 mg/L). By contrast, only 64% patients had measurable levels of monoclonal protein (≥200 mg per 24 hours) in urine protein electrophoresis (UPEP). After 1 and 3 treatment cycles, iFLC remained elevated in 71% and 46% of patients, respectively, whereas UPEP reported a positive result in 37% and 18%; all of the patients with positive UPEP at cycle 3 also had elevated iFLC levels. Importantly, elevated iFLC or an abnormal κ:λ sFLC ratio after 3 treatment cycles associated with poorer progression-free survival (P = .006 and P < .0001, respectively), whereas positive UPEP or urine immunofixation electrophoresis (uIFE) did not. In addition, patients with an abnormal κ:λ sFLC ratio had poorer overall survival (P = .022). Finally, early normalization of κ:λ sFLC ratio but not negative uIFE predicted achieving negative minimal residual disease, as determined by flow cytometry, after consolidation therapy (100% positive predictive value). We conclude that improved sensitivity and prognostic value of serum over urine measurements provide a strong basis for recommending the former for monitoring LCMM patients.
Medscape Continuing Medical Education online
This activity has been planned and implemented through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 3014.
Disclosures
Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Pfizer. Editor Nancy Berliner received grants for clinical research from Novartis. Author Olivier Decaux received lecture fees, a travel grant, and a supply of free reagents from Sebia, the Binding Site, and Siemens.
Learning objectives
Compare serum free light chain (sFLC) and involved (monoclonal) FLC (iFLC) in serum vs urine protein electrophoresis (UPEP) as a diagnostic disease marker in light-chain multiple myeloma (LCMM) on the basis of an analysis from the Intergroupe Francophone du Myélome (IFM)-2009 trial.
Compare iFLC in serum vs UPEP as a marker of treatment response evaluation in LCMM on the basis of an analysis from the IFM-2009 trial.
Determine other advantages of serum iFLC vs UPEP and possible reasons for its superiority as a disease marker on the basis of an analysis from the IFM-2009 trial.
Release date: December 22, 2016; Expiration date: December 22, 2017
Introduction
Measurement of immunoglobulin free light chains (FLCs) in patient serum became a practical option with the commercial availability of polyclonal, nephelometric assays in 2001.1 Serum assays offer many advantages over the traditional, electrophoretic, urine FLC assays2-4 and have been used widely as an aid for the diagnosis and management of myeloma patients. The International Myeloma Working Group (IMWG) guidelines currently recommend the use of serum FLC (sFLC) assays in diagnostic screening panels and as a “biomarker of malignancy” (involved-to-uninvolved sFLC ratio ≥100) defining the presence of multiple myeloma (MM) in the absence of other indications.5
However, for response assessment throughout the course of treatment, the IMWG only recommend sFLC measurement for patients in whom other markers are below concentrations considered to be reliably measurable, that is, ≤10 g/L serum monoclonal protein and ≤200 mg per 24 hours urine monoclonal protein.6 In the same guidelines, response is defined as “measurable” with sFLC assays if the “involved” FLC (ie, the FLC class produced by the tumor) is at a serum concentration of ≥100 mg/L. The recommendation for patients with light-chain MM (LCMM) and measurable FLC in both serum and urine is, therefore, the preferential use of urine measurements for routine response assessment.
Urinary FLC measurement is known to have a number of drawbacks, including the practical difficulties of obtaining accurate 24-hour urine collections from patients who may be elderly and frail.7,8 Additionally, the interpretation and measurement of protein bands on electrophoresis gels can be subjective and inaccurate at low concentrations.9,10 Most important, however, is the efficient reabsorption and catabolism of FLC in the proximal tubules of the kidney11-13 such that measurable FLC in the urine represents overflow proteinuria and can be influenced by renal status as much as tumor production of FLC. A direct consequence of the renal metabolism of FLC is that sFLC assays are routinely more sensitive than renal assays for the identification of abnormal FLC production and this has been reported in many comparison studies.14-18 For the same reason, serial FLC measurements in serum and urine exhibit poor correlation; in a study of 399 MM patients, Dispenzieri and colleagues reported that the correlation between the 2 assay systems was insufficient to consider the tests interchangeable.19 It is, undoubtedly, this latter consideration that has led to the continued prioritization of urine assays for monitoring in the IMWG guidelines. Taking a different viewpoint, the current British Committee for Standards in Haematology (BCSH) guidelines comment that there is a “clear rationale” for using sFLC assays to assess response in LCMM patients, irrespective of the extent of light-chain excretion in the urine.20
In the present study, a large cohort of LCMM patients, treated in an Intergroupe Francophone du Myélome (IFM) MM trial, had both urine FLC and sFLC measurements recorded. Response assessments, based on the different methodologies were contrasted and compared with clinical outcomes. We propose that the data presented here, in conjunction with that from previously published studies, is sufficient to support a recommendation for monitoring and assessing LCMM patients’ response using sFLC in preference to urinary measurements.
Patients and methods
Patients
Between November 2010 and December 2012, 700 newly diagnosed MM patients, under the age of 66 years, were enrolled into the IFM 2009 trial (clinicaltrials.gov identifier NCT01191060). Patients were randomized to either arm A, which included 8 treatment cycles of lenalidomide, bortezomib, and dexamethasone (RVD), or arm B, which included 3 cycles of RVD followed by high-dose melphalan (with autologous stem cell rescue) and 2 further RVD consolidation cycles. All patients received 1 year of lenalidomide maintenance therapy. Exclusion criteria were the presence serum creatinine >25 mg/L or a creatinine clearance of <60 mL per minute. Of the 700 patients in the therapeutic trial, 113 patients with LCMM were included in the present study (patients with an intact immunoglobulin detected only by serum immunofixation were excluded).
Laboratory methods
Urine protein electrophoresis (UPEP) and urine immunofixation electrophoresis (uIFE; Sebia) were performed prospectively throughout the trial, using standard laboratory procedures on neat samples. sFLC concentrations were measured using κ sFLC and λ sFLC Freelite assays (The Binding Site Group Ltd) on a BN II nephelometer (Siemens). Reference ranges for κ sFLC were 3.3 to 19.4 mg/L; for λ sFLC, 5.7 to 26.3 mg/L; and for the κ:λ sFLC ratio, 0.26 to 1.65 (referred to hereafter as sFLC ratio for ease of reading). Involved (monoclonal) FLC (iFLC) refers to the concentration of the tumor-derived light chain. Urines at the end of cycle 1 were assessed locally across participating IFM centers. All other measurements were done at our central laboratory in University Hospital Nantes (Nantes, France).
Response assessment
The definitions of measurable disease were assigned according to IMWG criteria.6 Comparison of the response assessment according to urine electrophoresis vs sFLC measurement was achieved by (1) comparing the number of patients who had become negative by UPEP with those whose iFLC concentrations had fallen within the normal range and (2) comparing the number of patients who had become negative by uIFE with those whose sFLC ratio was within the normal range. These assessments approximate to the IMWG criteria of very-good-partial response and complete response (CR)/stringent CR, respectively. The IMWG criteria for CR could not strictly be applied as that would have required bone marrow plus plasmacytoma screening; these data have not been recorded at cycles 1 and 3 for all patients included in the analyses. The comparisons were made between responses evaluated after the first cycle of RVD treatment and at the end of induction therapy (3 cycles of RVD).
Minimal residual disease (MRD) negativity was determined by flow cytometry using a 7-color antibody panel with a 10−4 sensitivity as previously reported, after the patients had received consolidation therapy.
Statistical analysis
Median follow-up was calculated using the reverse Kaplan-Meier method and median time to progression and death was calculated using Kaplan-Meier estimates. Agreement between UPEP and FLC test results after 1 cycle and after 3 cycles of treatment were looked at separately. It is important to note that, where UPEP was not tested at the visit after 1 or 3 cycles of treatment but test results shortly before and after (±21 days) are the same (either both positive or negative), it was assumed that the missing value for UPEP was the same and therefore “imputed” the test result at the time point of interest. For comparative studies, only patients with matched urine and sFLC test results were included for each analysis. Differences in progression-free survival (PFS) and overall survival (OS) between patient groups were analyzed using Kaplan-Meier survival curves with the log-rank test used to indicate significance. Investigating the association of variables with PFS and OS was carried out with a Cox proportional hazard model. Significant differences between categorical variables were calculated with the χ2 test. Statistical analyses were performed using SPSS v23 (IBM). Survival graphs were generated using GraphPad/Prism 5 software.
Ethical considerations
The study was approved by the local ethics committee and conducted in agreement with the Declaration of Helsinki and Good Clinical Practice Guidelines. Written informed consent from participating patients was required.
Results
Median follow-up duration was 3.4 years (95% confidence interval [CI], 3.3-3.7 years). Median time to progression was 4.2 years (95% CI, 3.5-4.9 years). Median time to death could not be calculated as the survival probability remained above 80% at the end of follow-up.
UPEP and FLC testing occurred at a median time of 31 days from baseline (range, 21-46 days) following 1 cycle of treatment and at a median of 84 days (range, 66-137 days) following 3 cycles of treatment.
The baseline characteristics for the 113 LCMM patients who were in this study are presented in Table 1, including a comparison of the UPEP and iFLC results. There were more patients with abnormal serum iFLC (median, 1890 [111-27 400] mg/L) than positive UPEP (median, 1350 [10-11 400] mg per 24 hours; 10 patients had a positive UPEP result with bands that were not quantifiable), and more patients with measurable sFLC concentrations for monitoring (ie, iFLC ≥100 mg/L) than measurable urine Bence Jones protein (≥200 mg per 24 hours).
Table 1.
Baseline characteristics of patients (n = 113)
| n (%) | Median (range) | |
|---|---|---|
| Age, y | 59 (29-66) | |
| Albumin, g/L | 42.3 (31.8-55.5) | |
| β2-microglobulin, mg/L | 2.9 (1.2-20.6) | |
| ISS I | 65 (58)* | |
| ISS II | 30 (27)* | |
| ISS III | 18 (16)* | |
| uIFE positive | 113 (100) | |
| κ | 72 (64) | |
| λ | 41 (36) | |
| UPEP positive | 87 (78)† | 1 350 (10-11 400)‡ |
| UPEP ≥200 mg per 24 h | 71 (64)† | |
| sFLC ratio abnormal | 113 (100) | |
| κ | 72 (64) | 400.6 (8.3-11 292) |
| λ | 41 (36) | 0.002 (0.0002-0.066) |
| iFLC, mg/L | 1 890 (111-27 400) | |
| iFLC ≥100 mg/L | 113 (100) | |
| Follow up, y | 3.4 (0.4-4.6) |
ISS, International Staging System.
Percentage totaling 101% due to rounding up.
Two patients missing data.
Milligrams per 24 hours.
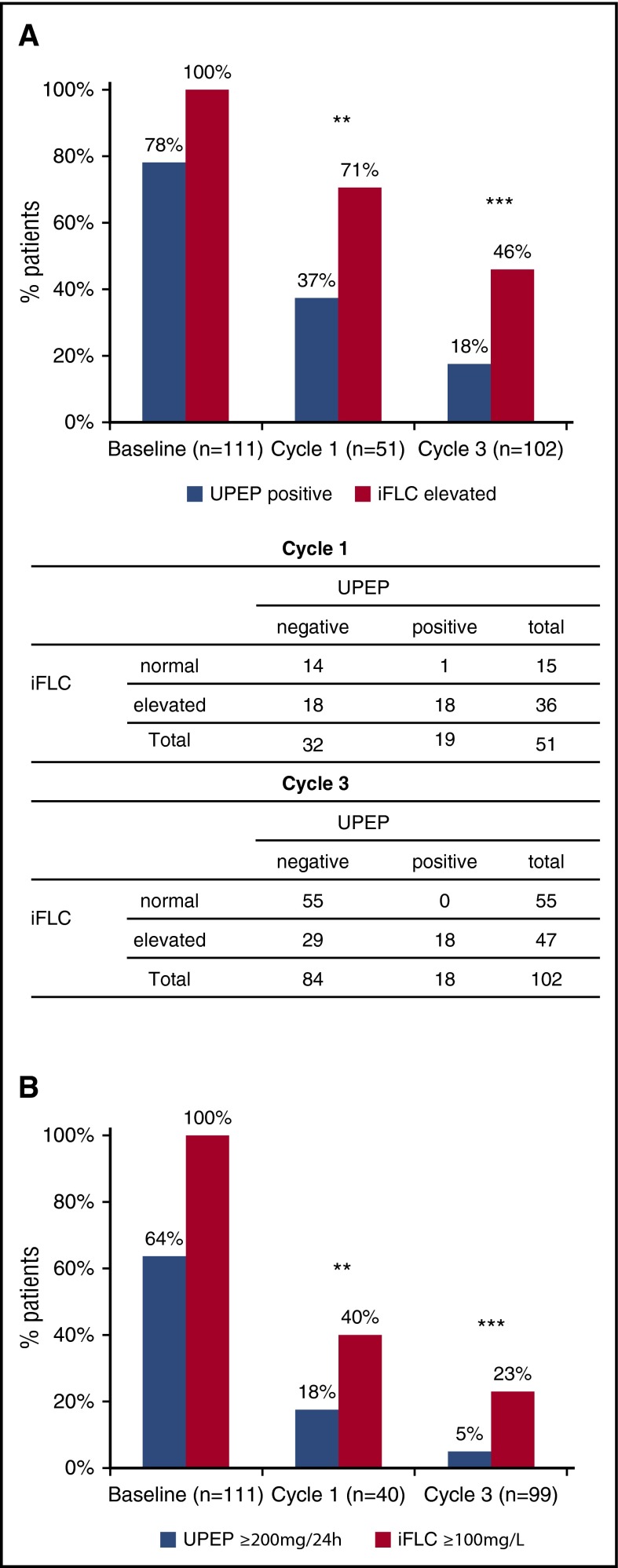
Figure 1 shows the percentage and number of patients with matched serum and urine data who were positive by UPEP or iFLC (Figure 1A) or “measurable” (according to IMWG definitions) by the same assays (Figure 1B), at baseline and after 1 and 3 cycles of treatment. It was notable that 100% (111 of 111) of the patients were abnormal by iFLC at baseline, whereas only 78% (87 of 111) were positive by UPEP, and that 100% had measurable disease by iFLC compared with 64% (71 of 111) for UPEP. These percentages fell, for both assays, after 1 and 3 cycles of treatment but the serum assay was the more sensitive at each time point. Patients’ percentage reductions between cycles by either method were statistically significant (P ≤ .009 for all comparisons). At all 3 time points, more patients were excluded from the analysis due to missing urine analysis than missing serum analysis, that is, at baseline, 2 missing urine vs 0 missing serum; cycle 1, 60 vs 7; and cycle 3, 11 vs 1.
Figure 1.
Sensitivity of urine FLC and sFLC measurements. (A) Percentage of patients with positive UPEP and elevated serum iFLC at baseline, and after 1 and 3 cycles of treatment (P = .004 and P < .001, respectively; baseline not evaluable as 100% patients had elevated iFLC). Below are comparative tables with number of patients identified by either test at cycles 1 and 3. (B) Percentage of patients with measurable levels of disease as determined by UPEP (≥200 mg per 24 hours) and serum iFLC (≥100 mg/L) measurements at baseline, and at end of cycles 1 and 3 (P = .007 and P < .001, respectively; baseline not evaluable as 100% patients had measurable disease by iFLC). P values calculated by χ2 test. Comparisons at each time point include patients with matched urine and serum data.
There were 18 and 29 patients abnormal by iFLC but negative by UPEP after 1 and 3 cycles, respectively. Only 1 patient was recorded as being positive by UPEP but having a normal iFLC concentration (after 1 cycle of treatment); however, the UPEP for this patient was recorded as <200 mg per 24 hours and total urine protein at 110 mg per 24 hours, casting some doubt about whether there was sufficient monoclonal FLC for a true positive UPEP result. It was also noted that in 58 patients with measurable disease at baseline by urine measurements (≥200 mg per 24 hours) and matched data after 3 cycles of treatment, 53 of 58 patients (91%) had become nonmeasurable by urine at the end of the 3 cycles; by contrast, 39 of 58 patients (67%) had become nonmeasurable by serum, that is, in 14 patients with nonmeasurable urine levels at the end of induction, the sFLC concentrations were still deemed as measurable. All 5 of 58 patients with measurable urine levels at cycle 3 also had sFLC levels >100 mg/L.
In addition, the percentage of patients with positive UPEP was smaller than with elevated iFLC at all of the time points analyzed during induction therapy. Specifically, the percentage of positive patients at baseline, cycle 1, and cycle 3 was 78, 37, and 18 for urinalysis but 100, 71, and 46 for iFLC elevation, respectively.
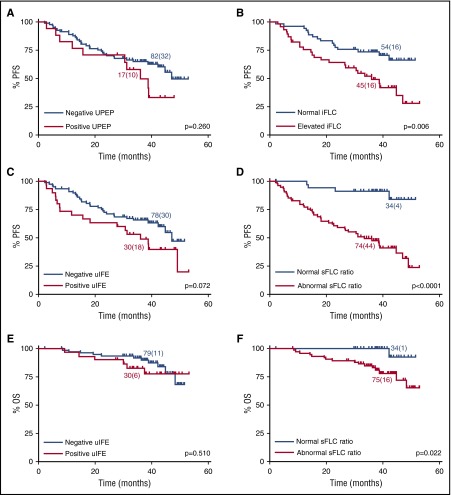
Figure 2A-D contains Kaplan-Meier graphs of PFS for patients grouped according to their UPEP and iFLC assessments (Figure 2A-B) and their uIFE and sFLC ratio results (Figure 2C-D). The assessments were made after 3 cycles of treatment. With both comparisons, the curve separation was better for the serum measures and the difference between the populations reached significance (log-rank analysis), whereas it did not for the urine assessments. Similar results were obtained for prediction of OS except that, in this instance, only the sFLC ratio results reached statistical significance (Figure 2E-F).
Figure 2.
Survival outcomes according to urine FLC and sFLC characteristics at the end of induction therapy. PFS for patients with (A) negative vs positive UPEP (median PFS, 47 and 36 months, respectively), (B) normal vs elevated iFLC (median PFS, not reached and 36 months, respectively), (C) negative vs positive uIFE (median PFS, 47 and 36 months, respectively), and (D) normal vs abnormal κ:λ sFLC ratio (median PFS, not reached and 34 months, respectively). OS for patients with (E) negative vs positive uIFE (median OS not reached for both) and (F) normal vs abnormal κ:λ sFLC ratio (median OS not reached for both). P values calculated by log-rank test. Number of patients (events) for each arm is indicated. Comparisons include patients with matched urine and serum data.
Cox regression univariate analysis of the same data produced greater hazard ratios and statistical significance for the serum results, whereas the urine results did not associate with outcome in any case (Table 2). Furthermore, calculation of the Akaike information criterion indicated that the sFLC models had a better fit, whereas calculation of the C statistic indicated better discrimination by the serum-based prognosis.
Table 2.
Cox regression univariate analysis and model fit
| HR | 95% CI | P | AIC | C statistic (95% CI) | |
|---|---|---|---|---|---|
| PFS | |||||
| Cycle 1 | |||||
| UPEP positive | 1.3 | 0.6-3.2 | .519 | 151.9 | 0.5 (0.4-0.6) |
| iFLC elevated | 5.0 | 1.2-21.3 | .032 | 145.4 | 0.6 (0.5-0.7) |
| uIFE positive | 1.1 | 0.6-2.4 | .731 | 245.1 | 0.5 (0.5-0.6) |
| sFLC ratio abnormal | 7.5 | 1.0-54.7 | .048 | 237.5 | 0.6 (0.5-0.6) |
| Cycle 3 | |||||
| UPEP positive | 1.6 | 0.8-3.3 | .178 | 351.9 | 0.5 (0.5-0.6) |
| iFLC elevated | 2.3 | 1.3-4.4 | .008 | 346.3 | 0.6 (0.5-0.7) |
| uIFE positive | 1.7 | 1.0-3.1 | .077 | 405.8 | 0.6 (0.5-0.6) |
| sFLC ratio abnormal | 6.8 | 2.5-19.0 | <.001 | 386.3 | 0.7 (0.6-0.7) |
| Postconsolidation | |||||
| iFLC elevated | 2.7 | 1.4-5.4 | .004 | — | — |
| sFLC ratio abnormal | 3.1 | 1.4-6.8 | .006 | — | — |
| OS | |||||
| Cycle 1 | |||||
| UPEP positive | 0.3 | 0.04-2.6 | .314 | 49.6 | 0.6 (0.5-0.7) |
| iFLC elevated* | — | — | — | 45.3 | 0.7 (0.6-0.7) |
| uIFE positive | 2.4 | 0.5-11.3 | .270 | 76.6 | 0.6 (0.5-0.7) |
| sFLC ratio abnormal* | — | — | — | 72.5 | 0.6 (0.5-0.6) |
| Cycle 3 | |||||
| UPEP positive | 0.9 | 0.2-4.0 | .891 | 116.0 | 0.5 (0.4-0.6) |
| iFLC elevated | 2.2 | 0.7-6.6 | .164 | 114.0 | 0.6 (0.5-0.7) |
| uIFE positive | 1.4 | 0.5-3.8 | .537 | 145.8 | 0.6 (0.5-0.7) |
| sFLC ratio abnormal | 7.8 | 1.0-58.5 | .047 | 139.0 | 0.7 (0.6-0.7) |
Bold values in the table body represent statistically significant results.
—, results not reported; AIC, Akaike information criterion; HR, hazard ratio.
None and 1 event were reported for patients with normal iFLC and FLC ratio, respectively, resulting in broad CIs.
The discriminatory power of UPEP and iFLC measurements was further contrasted by the analyses illustrated in Figure 3A-B. After 3 cycles of treatment, the 82 patients who were negative by UPEP could be separated into 2 groups based upon normal/elevated iFLC concentrations, and those with elevated iFLC had significantly shorter PFS (Figure 3A). There were no patients with normal iFLC and positive UPEP; however, for the 45 patients with elevated iFLC, separation according to positive/negative UPEP was not prognostic of PFS (Figure 3B). Figure 3C-D contrasts the predictive power of the uIFE and sFLC ratio recorded after 3 treatment cycles. Of the patients negative by uIFE, separation according to normal vs abnormal sFLC ratio was predictive of PFS (P < .0001) and also of OS (P = .016). At this time point, there were no patients with normal sFLC ratio and a positive uIFE.
Figure 3.
Abnormal sFLC measurements stratify patients with normal urine results after induction. PFS according to (A) normal vs elevated iFLC in patients with a negative UPEP result (median PFS, not reached and 34 months, respectively), (B) negative vs positive UPEP in patients with elevated iFLC (median PFS, 34 and 36 months, respectively), and (C) normal vs abnormal κ:λ sFLC ratio in patients with negative uIFE (median PFS, not reached and 31 months, respectively). (D) OS for patients with normal vs abnormal κ:λ sFLC ratio in patients with negative uIFE (median OS not reached for both). P values calculated by log-rank test. Number of patients (events) for each arm is indicated. Comparisons include patients with matched urine and serum data.
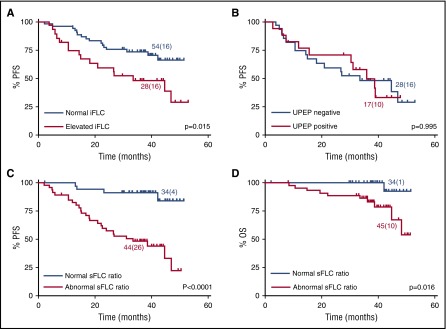
In addition, we evaluated the prognostic value of FLC measurements late during monitoring. Cox regression and Kaplan-Meier analyses demonstrated that both elevated iFLC levels (log-rank test: P = .003) and an abnormal sFLC ratio (P = .004) at the end of consolidation therapy (∼8 months into the trial protocol) maintained their prognostic value and associated with shorter PFS (Table 2; Figure 4).
Figure 4.
Survival outcomes according to sFLC characteristics at end of consolidation therapy. PFS for patients with (A) normal vs elevated iFLC (median PFS, not reached and 31 months, respectively) and (B) normal vs abnormal κ:λ sFLC ratio (median PFS, not reached and 40 months, respectively). P values calculated by log-rank test. Number of patients (events) for each arm is indicated.
We assessed the value of achieving uIFE negativity or a normal sFLC ratio during induction therapy for the prediction of MRD negativity at the end of consolidation treatment. In patients with matched data for serum, urine, and MRD assessment, normalization of sFLC ratio after both 1 and 3 treatment cycles had 100% positive predictive value (PPV), that is, all patients whose sFLC ratios normalized during induction went on to achieve MRD negativity postconsolidation (Table 3); by contrast, patients becoming uIFE-negative at cycles 1 and 3 had PPVs of 81% and 78%, respectively.
Table 3.
PPV of serum and urine early normalization (cycles 1 and 3) for MRD response (by flow cytometry) postconsolidation
| Method | Method result | Achieving MRD negativity | |||
|---|---|---|---|---|---|
| Yes | No | PPV, % | |||
| Cycle 1 (n = 43) | uIFE negative | Yes | 17 | 4 | 81 |
| No | 12 | 10 | |||
| sFLC ratio normal | Yes | 9 | 0 | 100 | |
| No | 20 | 14 | |||
| Cycle 3 (n = 66) | uIFE negative | Yes | 40 | 11 | 78 |
| No | 5 | 10 | |||
| sFLC ratio normal | Yes | 22 | 0 | 100 | |
| No | 23 | 21 | |||
Discussion
The comparison of UPEP and iFLC results at the time of diagnosis (baseline) showed that iFLC was a more sensitive measure of disease, with 100% of patients positive and “measurable” compared with 78% positive and 64% measurable by UPEP. This pattern of results was repeated with the assessments after 1 and 3 cycles of treatment and was also in accord with all other published studies of patients with confirmed diagnoses of LCMM.14,21 UPEP and iFLC were chosen as the measures for comparison as they are the methods approved for monitoring LCMM by the IMWG6 and are quantitative, in contrast to the more sensitive but nonquantitative uIFE or the more sensitive sFLC ratio which may become variable if the noninvolved light chain is highly suppressed.19 Nevertheless, achieving a normal sFLC has been shown to be a clinically relevant measure of treatment efficacy.22,23
The number of missing observations was much higher for the urine assays, particularly after cycle 1, with 60 missing, although urine compliance improved at subsequent collection times throughout the protocol. The limited numbers did reduce the statistical certainty achieved in any analyses (resulting in broad CIs) and also serve to illustrate the practical difficulties encountered collecting urine samples even when patients were participating in a trial.24,25
Another notable feature of the results was the 14 patients whose urine Bence Jones protein concentrations fell at the end of induction below the concentration regarded as measurable (≥200 mg per 24 hours) by IMWG guidelines,6 while maintaining measurable levels of sFLC. For these patients, adherence to the current guidelines for monitoring would dictate that they were initially monitored using urine electrophoresis but then transferred to serum monitoring. Although trends in FLC concentrations are generally consistent between sFLC and urine FLC assessments (ie, both assessments show falling, or rising, concentrations), the absolute concentration in urine shows little correlation with that in serum,19 so changing the method of monitoring is less than ideal and creates a potential source of confusion. Obviously, these difficulties could be avoided if the patients were assessed by their sFLC throughout their treatment.
With regard to the urine FLC and sFLC response assessments after 1 and 3 cycles of treatment, the urine measurements clearly indicated a generally greater degree of response, with more patients becoming negative or nonmeasurable by urine analysis. The most obvious and simple interpretation of these results is that the urine assays underestimated the amount of FLC production and overestimated the response to treatment because of the reabsorption and metabolism of FLC in the kidneys. The assumption that sFLC concentrations may provide a more true reflection of the disease process is supported by the fact that they were more predictive of PFS and OS. Bradwell et al reported in an early MM trial14 in the United Kingdom that the percentage of LCMM patients achieving CR was 32% according to urine assessments and only 10% by sFLC, but the latter percentage was in much better accord with the 11% of intact immunoglobulin multiple myeloma (IIMM) patients (treated under the same protocol) judged to reach CR by electrophoretic serum assays. Likewise, in an interim analysis of the current IFM trial, Corre et al reported that the proportion of patients with normal iFLC levels at the end of induction was comparable between LCMM (52%) and IIMM (58%) patients, and closer to the 21% of IIMM patients whose serum protein electrophoresis was negative at the same time point; by contrast, UPEP was negative in 79% of LCMM patients at the end of induction, highlighting overestimation of the response by urine compared with serum measurements.26
Kaplan-Meier survival analyses showed that patients with an abnormal (elevated) iFLC had a significantly shorter PFS than patients with normal iFLC. However, the data from UPEP (positive/negative) did not reach significance. Further statistical analysis of the results also indicated that the model fit was better with the serum data as well as providing better discrimination for patient outcome. Although the number of events was too low for the data to reach significance for OS (unless the larger, unmatched iFLC data set was used; data not shown) all indications were that the serum iFLC measurements were prognostic of outcome. Interestingly, although the sFLC ratio was found to be more prognostic than uIFE (for PFS and OS), it was also more prognostic than iFLC measurements. This was indicated by the fact that the FLC ratio was significantly predictive of OS even when the smaller patient group (with matched serum and urine results) was analyzed.
It is relevant to note that sFLC measurements have previously been found to be prognostic for progression of monoclonal gammopathy of undetermined significance27,28 and smoldering MM.29,30 For LCMM, a study of 122 patients treated in a Medical Research Council myeloma trial in the United Kingdom indicated that normalization of iFLC concentrations and FLC ratios was prognostic of improved PFS and OS.31 Our results expand these observations and confirm the prognostic utility of sFLC measurements, both in early responders as well as in those patients whose sFLC parameters normalize later during monitoring. Likewise for IIMM, abnormal sFLC has been reported to be prognostic at presentation and during early monitoring in a number of studies,32-34 whereas the survival benefit of a CR including normalization of the sFLC ratio has been demonstrated22,23 and stringent CR is established as a response category in the IMWG guidelines.6 Similar prognostic utility has not been proposed for urine assessments.
These results raise the question of why the concentration of FLC in the urine is less prognostic than the concentration in the serum? One explanation might be that the renal “threshold” (ie, the sFLC concentration above which significant amounts of FLC pass into the urine) is very variable, such that some patients, with relatively low FLC (tumor) production, may still have high urine FLC concentrations. Conversely, patients with very efficient renal metabolism could have undetectable concentrations of FLC in the urine despite high levels of FLC production. Certainly, the comparison of sFLC and urine FLC results by Nowrousian et al indicated great variability for the renal threshold.15
MRD negativity for MM patients is increasingly believed to be a desirable and achievable aim of therapy.35 In the current (LCMM) study, all patients whose sFLC ratio normalized after 1 or 3 treatment cycles went on to achieve MRD negativity. This further underlines the clinical relevance of sFLC responses and suggests that routine sFLC monitoring could be a useful aid in guiding treatment.
In summary, the practical difficulties of obtaining 24-hour urine collections are well known and were also illustrated in the current study, whereas serum samples are much more readily obtained. In keeping with previous reports, sFLC analysis was found to be a more sensitive indicator of disease than urinalysis at all time points evaluated, thus demonstrating that monitoring LCMM patients with serum assays would minimize the need to change techniques. Importantly, and to the best of our knowledge, we demonstrate for the first time that the improved sensitivity of serum measurements translates into valuable prognostic information, adding to the existing evidence for the greater biological relevance of sFLC measurement.
sFLC analysis is already recommended at diagnosis and maximum response; it would appear sensible to use the same methodology at the time points in between. We believe there is now sufficient evidence and experience to propose that sFLC analysis is the method of choice for response evaluation in LCMM patients, whereas urine exploration may remain a rational practice to assess total proteinuria and, together with serum creatinine measurements, monitor renal function in these patients.
Acknowledgments
The authors thank all of the patients, families, nurses, study coordinators, and support staff who contributed to this study. The Binding Site Group Ltd provided Freelite kits free of charge. Kym Snell (University of Birmingham, Birmingham, United Kingdom) provided statistical data analysis assistance.
This work was supported by the Cancer Pharmacology of Toulouse-Oncopole and Region program, a National Institutes of Health, National Cancer Institute PO1 grant (CA100707-12), and Fondation ARC pour la recherche sur le cancer (labelized team).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.A.-L. designed the research; T.D. performed sample analysis; H.A.-L., T.D., P.M., and M.A. wrote the paper; and all of the authors contributed with recruitment of patients and samples.
Conflict-of-interest disclosure: O.D. received lecture fees, a travel grant, and a supply of free reagents from Sebia, the Binding Site, and Siemens. The remaining authors declare no competing financial interests.
Correspondence: Herve Avet-Loiseau, Institut Universitaire du Cancer, Toulouse, France and Centre de Recherche en Cancerologie de Toulouse, INSERM U1037, Avenue Hubert Curien, 31100 Toulouse, France; e-mail: avetloiseau.herve@iuct-oncopole.fr.
References
- 1.Bradwell AR, Carr-Smith HD, Mead GP, et al. . Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47(4):673-680. [PubMed] [Google Scholar]
- 2.Keren DF. Serum protein electrophoresis evaluation of monoclonal gammopathies (M-proteins). J Clin Ligand Assay. 2005;27(4):218-226. [Google Scholar]
- 3.Charafeddine KM, Jabbour MN, Kadi RH, Daher RT. Extended use of serum free light chain as a biomarker in lymphoproliferative disorders: a comprehensive review. Am J Clin Pathol. 2012;137(6):890-897. [DOI] [PubMed] [Google Scholar]
- 4.Pratt G. The evolving use of serum free light chain assays in haematology. Br J Haematol. 2008;141(4):413-422. [DOI] [PubMed] [Google Scholar]
- 5.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. . International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. [DOI] [PubMed] [Google Scholar]
- 6.Rajkumar SV, Harousseau JL, Durie B, et al. ; International Myeloma Workshop Consensus Panel 1. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel DS, McBride L, Bilotti E, et al. . Inaccuracies in 24-hour urine testing for monoclonal gammopathies. Lab Med. 2009;40(6):341-344. [Google Scholar]
- 8.Kaplan JS, Horowitz GL. Twenty-four-hour Bence-Jones protein determinations: can we ensure accuracy? Arch Pathol Lab Med. 2011;135(8):1048-1051. [DOI] [PubMed] [Google Scholar]
- 9.Levinson SS, Keren DF. Free light chains of immunoglobulins: clinical laboratory analysis. Clin Chem. 1994;40(10):1869-1878. [PubMed] [Google Scholar]
- 10.Keren DF. Protein Electrophoresis in Clinical Diagnosis. Boca Raton, FL: CRC Press; 2003. [Google Scholar]
- 11.Wochner RD, Strober W, Waldmann TA. The role of the kidney in the catabolism of Bence Jones proteins and immunoglobulin fragments. J Exp Med. 1967;126(2):207-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abraham GN, Waterhouse C. Evidence for defective immunoglobulin metabolism in severe renal insufficiency. Am J Med Sci. 1974;268(4):227-233. [DOI] [PubMed] [Google Scholar]
- 13.Maack T, Johnson V, Kau ST, Figueiredo J, Sigulem D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: a review. Kidney Int. 1979;16(3):251-270. [DOI] [PubMed] [Google Scholar]
- 14.Bradwell AR, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet. 2003;361(9356):489-491. [DOI] [PubMed] [Google Scholar]
- 15.Nowrousian MR, Brandhorst D, Sammet C, et al. . Serum free light chain analysis and urine immunofixation electrophoresis in patients with multiple myeloma. Clin Cancer Res. 2005;11(24 Pt 1):8706-8714. [DOI] [PubMed] [Google Scholar]
- 16.Avet-Loiseau H, Mirbahai L, Mathiot C, et al. . Comparison of serum free light chain ratios with standard urine analysis in diagnosis and monitoring of multiple myeloma. Haematologica. 2011;96(s1):P-075a. [Google Scholar]
- 17.Avet-Loiseau H, Young P, Mathiot C, et al. . Nephelometric measurements of κFLC and λFLC for monitoring light chain myeloma patients. Haematologica. 2011;96(s2):0853a. [Google Scholar]
- 18.Katzmann JA, Snyder MR, Rajkumar SV, et al. . Long-term biological variation of serum protein electrophoresis M-spike, urine M-spike, and monoclonal serum free light chain quantification: implications for monitoring monoclonal gammopathies. Clin Chem. 2011;57(12):1687-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dispenzieri A, Zhang L, Katzmann JA, et al. . Appraisal of immunoglobulin free light chain as a marker of response. Blood. 2008;111(10):4908-4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bird J, Owen R, D’Sa S, et al. . Guidelines for the diagnosis and management of multiple myeloma 2014. BCSH guideline. London, United Kingdom: British Committee for Standards in Haematology; 2014.
- 21.Dejoie T, Attal M, Moreau P, Harousseau JL, Avet-Loiseau H. Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma. Haematologica. 2016;101(3):356-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moustafa M, Rajkumar V, Dispenzieri A, et al. . Utility of serum free light chain measurements in multiple myeloma patients not achieving complete response [abstract]. Blood. 2014;124(21). Abstract 2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapoor P, Kumar SK, Dispenzieri A, et al. . Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. J Clin Oncol. 2013;31(36):4529-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holding S, Spradbery D, Hoole R, et al. . Use of serum free light chain analysis and urine protein electrophoresis for detection of monoclonal gammopathies. Clin Chem Lab Med. 2011;49(1):83-88. [DOI] [PubMed] [Google Scholar]
- 25.Robson EJD, Taylor J, Beardsmore C, Basu S, Mead G, Lovatt T. Utility of serum free light chain analysis when screening for lymphoproliferative disorders. Lab Med. 2009;40(6):325-329. [Google Scholar]
- 26.Corre J, Dejoie T, Caillon H, Attal M, Avet-Loiseau H, Moreau P. Serum free light chains should be the target of response evaluation in light chain multiple myeloma rather than urines: results from the IFM/DFCI 2009 trial [abstract]. Blood. 2014;124(21). Abstract 180a. [Google Scholar]
- 27.Rajkumar SV, Kyle RA, Therneau TM, et al. . Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106(3):812-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turesson I, Kovalchik SA, Pfeiffer RM, et al. . Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid malignancies: 728 cases followed up to 30 years in Sweden. Blood. 2014;123(3):338-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dispenzieri A, Kyle RA, Katzmann JA, et al. . Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111(2):785-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen JT, Kumar SK, Dispenzieri A, Kyle RA, Katzmann JA, Rajkumar SV. Serum free light chain ratio as a biomarker for high-risk smoldering multiple myeloma. Leukemia. 2013;27(4):941-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyle E, Brioli A, Leleu X, et al. . The value of serum free light chain monitoring compared to urinary Bence-Jones measurement in light chain only myeloma [abstract]. Blood. 2013;122(21). Abstract 1895a. [Google Scholar]
- 32.Kyrtsonis MC, Vassilakopoulos TP, Kafasi N, et al. . Prognostic value of serum free light chain ratio at diagnosis in multiple myeloma. Br J Haematol. 2007;137(3):240-243. [DOI] [PubMed] [Google Scholar]
- 33.van Rhee F, Bolejack V, Hollmig K, et al. . High serum-free light chain levels and their rapid reduction in response to therapy define an aggressive multiple myeloma subtype with poor prognosis. Blood. 2007;110(3):827-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snozek CL, Katzmann JA, Kyle RA, et al. . Prognostic value of the serum free light chain ratio in newly diagnosed myeloma: proposed incorporation into the international staging system. Leukemia. 2008;22(10):1933-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munshi NC, Anderson KC. Minimal residual disease in multiple myeloma. J Clin Oncol. 2013;31(20):2523-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]