Abstract
Background
We aimed to summarize the diagnostic accuracy of white light bronchoscopy (WLB) and advanced techniques for airway pre-cancerous lesions and early cancer, such as autofluorescence bronchoscopy (AFB), AFB combined with WLB (AFB + WLB) and narrow-band imaging (NBI) bronchoscopy.
Methods
We searched for eligible studies in seven electronic databases from their date of inception to Mar 20, 2015. In eligible studies, detected lesions should be confirmed by histopathology. We extracted and calculated the 2×2 data based on the pathological criteria of lung tumor, including high-grade lesions from moderate dysplasia (MOD) to invasive carcinoma (INV). Random-effect model was used to pool sensitivity, specificity, diagnostic odds ratio (DOR) and the area under the receiver-operating characteristic curve (AUC).
Results
In 53 eligible studies (39 WLB, 39 AFB, 17 AFB + WLB, 6 NBI), diagnostic performance for high-grade lesions was analyzed based on twelve studies (10 WLB, 7 AFB, 7 AFB + WLB, 1 NBI), involving with totally 2,880 patients and 8,830 biopsy specimens. The sensitivity, specificity, DOR and AUC of WLB were 51% (95% CI, 34–68%), 86% (95% CI, 73–84%), 6 (95% CI, 3–13) and 77% (95% CI, 73–81%). Those of AFB and AFB + WLB were 93% (95% CI, 77–98%) and 86% (95% CI, 75–97%), 52% (95% CI, 37–67%) and 71% (95% CI, 56–87%), 15 (95% CI, 4–57) and 16 (95% CI, 6–41), and 76% (95% CI, 72–79%) and 82% (95% CI, 78–85%), respectively. NBI presented 100% sensitivity and 43% specificity.
Conclusions
With higher sensitivity, advanced bronchoscopy could be valuable to avoid missed diagnosis. Combining strategy of AFB and WLB may contribute preferable diagnosis rather than their alone use for high-grade lesions. Studies of NBI warrants further investigation for precancerous lesions.
Keywords: Advanced bronchoscopy, lung cancer, white light bronchoscopy (WLB), autofluorescence bronchoscopy (AFB), narrow-band imaging bronchoscopy
Introduction
Lung cancer is one of the leading causes of cancer mortality worldwide (1), mainly attributed to its biologically aggressive nature and late stage at the time of diagnosis. However, the 5-year survival rate of patients stage IA could be up to 74.6% (2), indicating patients with longer life expectancy after diagnosis and treatment in early stage. A long-term follow-up (12.5 years) surveillance study found 34% lung-cancer detection rate in patients harboring endobronchial pre-invasive lesions after median 16.5 months (3), suggesting these patients at high risk of primary or secondary cancer. Therefore, diagnosis of precancerous lesions and early-stage lung cancer is crucial.
Currently, conventional white light bronchoscopy (WLB) is the most common tool for the detection of central-airway lung precancerous and cancerous lesions. In some cases, these lesions may be too thin or diminutive to be detected under the WLB. In order to address this limitation, advanced techniques such as autofluorescence bronchoscopy (AFB) and narrow-band imaging (NBI) bronchoscopy have been developed. AFB is the technique that emits fluorescent light containing green (520 nm peak) and red spectrum (>630 nm peak) (4), normal mucosa reflects this fluorescent light and presents a green-color image, while precancerous and cancerous lesions (even a few millimeters in diameter) absorb the green spectrum, and the reflected light turns magenta. NBI, another new-developed technique, only presents two narrow-bands of light (400–430 and 525–550 nm respectively) that can be absorbed by hemoglobin, in order to demonstrate a detailed image of the surface structure of lesions and superficial mucosal capillaries (5).
Though the superior diagnostic performance of AFB, AFB combined with WLB (AFB + WLB) and NBI (versus WLB) has been investigated for lung cancer in several comparison meta-analyses (6-9), all these available bronchoscopic techniques have not been overviewed for precancerous and cancer lesions [including high-grade lesions from moderate dysplasia (MOD) to INV] by single-arm synthesis in one article yet.
In this systematic review and meta-analysis, we primarily summarized the diagnostic accuracy of each technique based on eligible studies (single-arm synthesis), and secondly, we conducted an exploratory comparison between advanced techniques versus WLB directly only based on comparison studies (direct comparison). During above processes, techniques’ performance for different lesions was investigated, including their performance for high-grade lesions.
Methods
This meta-analysis was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (10). We performed an independent double-blind quality assessment (with J Zhang, blind to J Wu) and data extraction (with J Zhang blind to J Wu, Z Xu, Y Yang, and Z Liang). Any discrepancies were resolved by the discussion with W Liang.
Search strategy and study selection
Scopus, Embase, Web of Science, PubMed, ProQuest (scholarly journals), the Cochrane Library and Ovid (all EBM review) databases were searched from their date of inception to Mar 20, 2015. The retrieval formula was: [(fluorescence or autofluorescence or autofluorescence imaging) or (narrow band or narrow-band imaging)] and bronchoscopy (all field) (English). Duplicate articles were removed, and articles with inappropriate publication types were excluded, including reviews, systematic reviews, meta-analysis, case reports, letters, correspondences, comments, editorials, conference abstracts, erratum, short surveys, books or book chapters, and notes. In eligible studies, these advanced techniques should be investigated for diagnosing early lung cancer in the range of hyperplasia, metaplasia, dysplasia (mild/moderate/severe), carcinoma in situ (CIS) and INV, confirmed by histopathology. Sufficient data for constructing 2×2 contingency tables should be given in eligible studies.
Quality assessment
The quality of all included studies was assessed according to the quality assessment of diagnostic accuracy studies-2 (QUADAS-2) (11). Question 3 in domain 4 “Were all patients included in the analysis?” was revised as “Were all patients or biopsy specimens included in the analysis?”, since there were two types of analysis for 2×2 data construction (patient- or biopsy-based). The risk of bias and concern regarding applicability were scored as “high”, “low” and “unclear” according to the answers of questions. Based on these scores in each domain of the tool, we rated the quality for each study (high quality: “low risk” and “low concern” in all domains; low quality: at least one “high risk” or “high concern”; moderate quality: at least one “unclear risk” or “unclear concern”, without “high risk” or “high concern”).
Data extraction
The following characteristics of each study were extracted: author, year, type of analysis, total number of patients, and pathological results of biopsy specimens. During this process, the extracted data only reflected the data in the final statistical analysis of each included study, for example, if the number of patients enrolled in a study was not equal to the number of patients finally included in analysis, we would extract the data belonging to the final analysis.
2×2 contingency tables of true positive, false positive, false negative and true negative were constructed based on relevant calculation formula and given data from studies, such as sensitivity, specificity, positive predictive value and negative predictive value (12,13). We calculated the 2×2 data in two sets: the first set was based on the original pathological diagnostic criteria of lung tumor, within the range from hyperplasia to INV; the second set was based on the criteria from MOD to INV, which was regarded as high-graded lesions. During the above process, if we found the results of two techniques were lacking sufficient comparability in comparison studies, e.g., both positive results (true positive plus false negative) were not equal, their 2×2 data were only used for our single-arm synthesis, not for direct comparison.
Statistical analysis
We estimated the pooled sensitivity, specificity, diagnostic odds ratio (DOR), and the area under the receiver-operating characteristic curve (AUC), the index considering the pooled sensitivity and specificity together. Hierarchical summary receiver operating characteristic (HSROC) curves were plotted for the overall performance of each technique.
The test of heterogeneity with the value of I2 in meta-analysis is lack of sufficient reliability, due to the correlation between sensitivity and specificity-the variation of sensitivity would be mutually influenced by the variation of specificity (14). However, random-effect model was used to attenuate the effect of heterogeneity because we assumed its existence during the process of data synthesis. Moreover, we conducted a meta-regression to assess the effects of study period, quality (based on QUADAS-2) and type of analysis on the heterogeneity.
For bivariate model has taken the relation between sensitivity and specificity into account for the threshold effect (15), and there is no existing test perfectly matching our meta-analysis (14), we did not respectively estimate the threshold effect and publication bias.
Statistic procedures were conducted using STATA 13.0 (StataCorp, College Station, US). When there were only three corresponding studies in a group, the pooled sensitivity, specificity, DOR and AUC with 95% CI were calculated using Meta-DiSc 1.4 (XI Cochrane Colloquium, Barcelona, Spain). For direct comparison, we used Z test to estimate whether significant difference was indicated (P<0.05), which was completed in Excel 2011 (Microsoft, Seattle, US).
Results
Study identification, characteristics and quality assessment
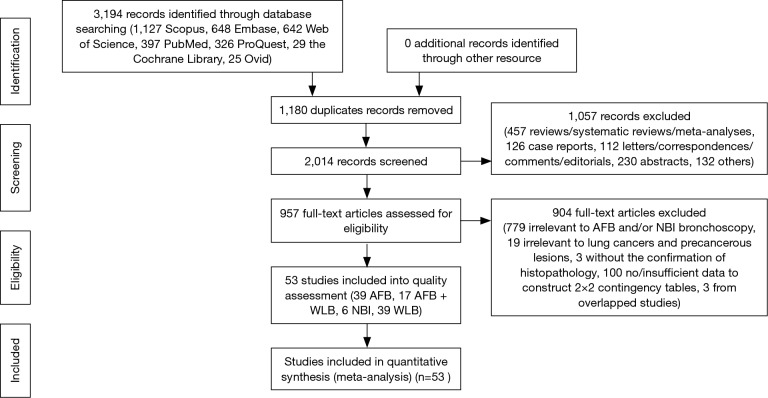
The details of study identification are shown in Figure 1. Three thousand one hundred and ninety-four articles were identified from electronic databases, of which 53 studies (39 WLB, 39 AFB, 17 AFB + WLB, 6 NBI) were finally included, involving a total of 6,543 patients and 18,458 biopsy specimens. Among these 53 included studies, twelve studies (10 WLB, 7 AFB, 7 AFB + WLB, 1 NBI) were within the data for the diagnosis of MOD to INV, involving 2,880 patients and 8,830 biopsy specimens. The characteristics of included studies are presented in Table 1, and detailed results of quality assessment are shown in Table S1.
Figure 1.
Flowchart of study selection. Three thousand one hundred and ninety-four studies were firstly identified. Finally, a total of 53 eligible studies were included in this meta-analysis. From: Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. For more information, visit www.prisma-statement.org.
Table 1. Characteristics of included studies.
| Author | Study quality | Type of analysis | Patient (n) | Biopsy (n) | Positive result of pathological criteria* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2×2 data | Total | HYP | MET | MIL | MOD | SEV | CIS | INV | |||||
| Kurie 1998 (16) | Moderate | Biopsy-based | 39 | 234 | Original& | 42 | – | 42 | – | – | |||
| Moro-Sibilot 2002 (17) | Moderate | Biopsy-based | 244 | 315 | Original | 42 | – | – | – | 23 | 19 | – | |
| Herth 2003 (18) | Moderate | Patient-based | 74 | Unclear | Original | 34 | – | – | – | – | – | 34 | |
| Lee 2007 (19) | High | Biopsy-based | 48 | 126 | Original | 22 | – | – | – | 5 | 13 | 4 | – |
| Moghissi 2008 (20) | Moderate | Patient-based | 93 | Unclear | Original | 20 | – | 20 | – | – | – | – | – |
| Lee 2009 (21) | High | Biopsy-based | 738 | 3,292 | Original | 278 | – | – | – | 87 | 99 | 92 | – |
| Zaric 2009 (22) | Moderate | Biopsy-based | 27 | 108 | Original | 40 | – | – | 4 | – | – | 36 | |
| Zaric 2010 (23) | High | Biopsy-based | 104 | 624 | Original | 309 | – | – | – | – | – | 309 | |
| Lam 1992 (24) | Moderate | Biopsy-based | 82 | 238 | Original | 37 | – | – | 16 | 12 | 6 | 3 | – |
| Lam 1993 (25) | Moderate | Biopsy-based | 94 | 264 | Original | 77 | – | – | – | 33 | 15 | 29 | – |
| Lam 1994 (26) | Moderate | Biopsy-based | 223 | 451 | Original | 113 | – | – | – | 78 | 35 | – | |
| Yokomise 1997 (27) | Moderate | Biopsy-based | 30 | 51 | Original | 20 | – | – | 4 | 16 | |||
| Horvath 1999 (28) | High | Biopsy-based | 56 | 146 | Original | 19 | 2 | 3 | 9 | 5 | – | – | – |
| Kakihana 1999 (29) | Moderate | Biopsy-based | 72 | 147 | Original | 79 | – | – | 51 | 28 | |||
| Ikeda 1999 (30) | High | Biopsy-based | 133 | 262 | Original | 127 | – | – | 72 | 55 | |||
| Weigel 1999 (31) | Moderate | Biopsy-based | 34 | 89 | Original | 6 | – | – | 3 | 3 | |||
| Weigel 2000 (32) | Moderate | Biopsy-based | 25 | 71 | Original | 4 | – | – | – | 3 | 0 | 1 | |
| Shibuya 2001 (33) | Moderate | Biopsy-based | 64 | 191 | Original | 45 | – | – | 42 | 3 | – | ||
| Furukawa 2000 (34) | Moderate | Biopsy-based | 108 | 234 | Original | 131 | – | – | 87 | 44 | |||
| Means-Markwell 2003 (35) | Moderate | Biopsy-based | 28 | 70 | Original | 2 | – | – | – | 1 | 0 | 1 | – |
| Lang 2005 (36) | High | Biopsy-based | 36 | 71 | Original | 26 | – | 5 | 2 | 0 | 19 | ||
| Chhajed 2005 (37) | High | Biopsy-based | 151 | 343 | Original | 83 | – | – | – | 52 | 8 | 3 | 20 |
| Chiyo 2005 (38) | Moderate | Biopsy-based | 32 | 60 | Original | 30 | – | – | 6 | 22 | 2 | – | – |
| Lam 2006 (39) | Moderate | Biopsy-based | 62 | 84 | Original | 12 | – | – | – | 5 | 4 | 2 | 1 |
| Ikeda 2006 (40) | Moderate | Biopsy-based | 154 | 166 | Original | 78 | – | – | 4 | 24 | 1 | 19 | 30 |
| Ueno 2007 (41) | High | Biopsy-based | 31 | 64 | Original | 30 | – | – | – | 11 | 2 | 1 | 16 |
| Nakanishi 2007 (42) | High | Biopsy-based | 71 | 288 | Original | 37 | – | – | – | – | 14 | 23 | |
| Gabrecht 2007 (43) | High | Biopsy-based | 21 | 41 | Original | 11 | – | – | 1 | 3 | 4 | 3 | |
| Li 2010 (44) | Moderate | Biopsy-based | 136 | 241 | Original | 76 | – | – | – | – | 3 | 0 | 73 |
| Ali 2011 (45) | High | Biopsy-based | 13 | 47 | Original | 13 | – | 4 | 2 | 7 | |||
| Cetýnkaya 2011 (46) | Moderate | Patient-based | 30 | 27 | Original | 7 | – | – | 6 | 1 | |||
| Venmans 1999 (47) | Moderate | Biopsy-based | 95 | 660 | Original | 79 | – | – | – | 31 | 39 | 9 | – |
| Kusunoki 2000 (48) | Moderate | Biopsy-based | 65 | 216 | Original | 49 | – | – | – | – | 21 | 9 | 19 |
| Hirsch 2001 (49) | High | Biopsy-based | 55 | 391 | Original | 71 | – | – | 71 (ASD) | – | – | ||
| Fuso 2005 (50) | High | Biopsy-based | 166 | 166 | Original | 93 | – | – | – | 13 | 80 | ||
| Ernst 2005 (51) | Moderate | Biopsy-based | 293 | 821 | Original | 85 | – | – | – | 85 | |||
| Divisi 2010 (52) | Moderate | Biopsy-based | 168 | 388 | Original | 328 | – | – | – | 328 | |||
| Lam 1998 (53) | High | Biopsy-based | 173 | 700 | Original | 142 | – | – | – | 93 | 9 | 40 | |
| Vermylen 1999 (54) | Moderate | Biopsy-based | 34 | 142 | Original | 16 | – | – | – | 7 | 3 | 6 | – |
| Häußinger 1999 (55) | Moderate | Biopsy-based | 60 | 264 | Original | 48 | – | – | 5 | 6 | 1 | 36 | |
| Extra# | 43 | – | – | – | 6 | 1 | 36 | ||||||
| Venmans 2000 (56) | Moderate | Biopsy-based | 59 | 267 | Original | 22 | – | – | – | 10 | 9 | 3 | – |
| Sato 2001 (57) | Moderate | Biopsy-based | 50 | 123 | Original | 67 | – | 39 | 28 | ||||
| Häußinger 2005 (58) | Moderate | Biopsy-based | 1,173 | 2,907 | Original | 53 | – | – | – | 19+34 | – | ||
| Hanibuchi 2007 (59) | Moderate | Biopsy-based | 123 | 282 | Original | 93 | – | 37 | 10 | 3 | 43 | ||
| Edell 2009 (60) | Moderate | Biopsy-based | 170 | 776 | Original | 76 | – | – | – | 33 | 6 | 2 | 35 |
| Xing 2005 (61) | Moderate | Biopsy-based | 95 | 200 | Original | 13 | – | – | – | 6 | 3 | 2 | 2 |
| Reinders 2009 (62) | Moderate | Biopsy-based | 367 | 749 | Original | 92 | – | – | – | – | 92 | ||
| Shibuya 2003 (63) | Moderate | Biopsy-based | 48 | 67 | Original | 15 | – | – | 15 (ASD) | – | – | ||
| Bojan 2009 (64) | Moderate | Biopsy-based | 36 | 132 | Original | 92 | – | 2 | – | – | – | 90 | |
| Zaric 2009 (65) | High | Biopsy-based | 106 | 636 | Original | 281 | – | – | – | – | – | 281 | |
| Vincent 2007 (66) | High | Biopsy-based | 22 | 64 | Original | 13 | – | – | – | 1 | 3 | 9 | |
| Nguyen 2013 (67) | Moderate | Biopsy-based | 70 | 64 | Original | 18 | – | – | – | 12 | 6 | – | – |
| Herth 2009 (68) | Moderate | Patient-based | 62 | 98 | Original | 17 | – | – | – | 17 | – | ||
*, the data we extracted would be only responsible for the final statistical analysis of each individual study; &, 2×2 data based on original pathological criteria used in included studies; #, 2×2 data based on pathological criteria from moderate dysplasia to invasive carcinoma, not used in included study. HYP, hyperplasia; MET, metaplasia; MIL, mild dysplasia; MOD, moderate dysplasia; SEV, severe dysplasia; CIS, carcinoma in situ; INV, invasive carcinoma; ASD, angiogenic squamous dysplasia.
Meta-analysis
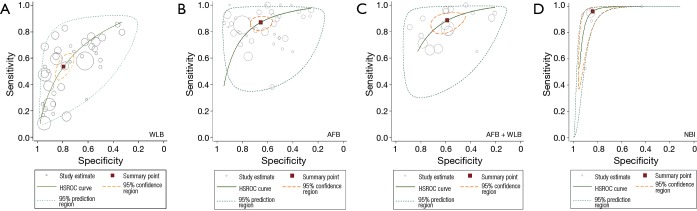
The performance of all techniques by single-arm synthesis was shown in Table 2. Based on original pathological criteria of different lung tumor, the sensitivity, specificity, DOR and AUC of WLB were 54% (95% CI, 46–61%), 79% (95% CI, 73–84%), 4 (95% CI, 3–6) and 72% (95% CI, 68–76%). The performance of AFB and AFB + WLB were close: 87% (95% CI, 82–90%) and 88% (95% CI, 82–93%) sensitivity, 60% (95% CI, 58–72%) and 59% (95% CI, 48–68%) specificity, 13 (95% CI, 8–19) and 11 (95% CI, 6–19) DOR, and 85% (95% CI, 81–87%) and 82% (95% CI, 78–85%) AUC. NBI presented remarkable diagnostic performance: 96% (95% CI, 78–99%) sensitivity, 84% (95% CI, 70–92%) specificity, 131 (95% CI, 35–490) DOR and 94% (95% CI, 91–96%) AUC. The HSROC of each technique were showed in Figure 2.
Table 2. Single-arm synthesis.
| Group | Study (n) | Patient (n) | Biopsy (n) | Sensitivity (95% CI) (%) | Specificity (95% CI) (%) | DOR | AUC (%) |
|---|---|---|---|---|---|---|---|
| Original pathological criteria | |||||||
| WLB | 39 | 4,356 | 11,587 | 54 [46–61] | 79 [73–84] | 4 [3–6] | 72 [68–76] |
| AFB | 39 | 4,027 | 11,149 | 87 [82–90] | 65 [58–72] | 13 [8–19] | 85 [81–87] |
| AFB + WLB | 17 | 3,208 | 9,150 | 88 [82–93] | 59 [48–68] | 11 [6–19] | 82 [78–85] |
| NBI | 6 | 344 | 1,061 | 96 [78–99] | 84 [70–92] | 131 [35–490] | 94 [91–96] |
| Moderate dysplasia, severe dysplasia, CIS and invasive carcinoma | |||||||
| WLB | 10 | 1,153 | 3,353 | 51 [34–68] | 86 [75–93] | 6 [3–13] | 77 [73–81] |
| AFB | 7 | 896 | 1,937 | 93 [77–98] | 52 [37–67] | 15 [4–57] | 76 [72–79] |
| AFB + WLB | 7 | 1,125 | 3,315 | 86 [75–97] | 71 [56–87] | 16 [6–41] | 82 [78–85] |
| NBI | 1 | 22 | 64 | 100 | 43 | – | – |
Data in parentheses are 95% CIs. WLB, white light bronchoscopy; AFB, autofluorescence bronchoscopy; AFB + WLB, AFB combined with WLB; NBI, narrow-band imaging; DOR, diagnostic odds ratio; AUC, area under the receiver-operating characteristic curve; CIS, carcinoma in situ.
Figure 2.
HSROC curve of WLB, AFB, AFB + WLB and NBI. The performance of NBI is remarkable, that of AFB and AFB + WLB is close. WLB showed the worse performance in single-arm synthesis. HSROC, hierarchical summary receiver operating characteristic; WLB, white light bronchoscopy; AFB, autofluorescence bronchoscopy; AFB + WLB, AFB combined with WLB; NBI, narrow-band imaging.
Based on the criteria of MOD to INV, majorities of diagnostic outcome were similar to the outcome based on original pathological criteria, except the specificity of AFB + WLB increased to 71% (56–87%), the AUC of AFB decreased to 76% (72–79%), and the specificity of NBI decreased to 43% (Table 2).
In the secondary part, we conducted an exploratory comparison between WLB and advanced bronchoscopies (Table 3). Based on original pathological criteria, though significant higher specificity of WLB was shown (P<0.05), its sensitivity (P<0.001), DOR (versus AFB: P<0.001; versus AFB + WLB: P=0.126; versus NBI: P=0.008) and AUC (versus AFB: P<0.001; versus AFB + WLB: P=0.083; versus NBI: P<0.030) were lower than those of three advanced techniques.
Table 3. Direct comparison.
| Group | Study (n) | Patient (n) | Biopsy (n) | Technique | Sensitivity (95% CI) (%) | P | Specificity (95% CI) (%) | P | DOR (95% CI) | P | AUC (95% CI) (%) | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Original pathological criteria | ||||||||||||
| WLB versus AFB | 30 | 2,492 | 6,062 | WLB | 51 [42–60] | <0.001 | 79 [72–85] | 0.002 | 4 [3–5] | <0.001 | 71 [67–75] | <0.001 |
| AFB | 86 [82–90] | 62 [54–70] | 10 [7–15] | 84 [81–87] | ||||||||
| WLB versus AFB + WLB | 14 | 2,578 | 7,813 | WLB | 51 [35–66] | <0.001 | 83 [75–89] | <0.001 | 5 [3–9] | 0.126 | 77 [74–81] | 0.083 |
| AFB + WLB | 88 [80–93] | 57 [45–68] | 10 [5–19] | 82 [78–85] | ||||||||
| WLB versus NBI | 3 | 154 | 226 | WLB | 29 [17–44] | <0.001 | 82 [74–88] | 0.043 | 2 [1–6] | 0.008 | 66 [47–85] | 0.030 |
| NBI | 79 [65–90] | 71 [62–78] | 19 [7–52] | 89 [82–96] | ||||||||
| Moderate dysplasia, severe dysplasia, CIS and invasive carcinoma | ||||||||||||
| WLB versus AFB | 6 | 728 | 1,549 | WLB | 50 [25–75] | 0.009 | 83 [64–93] | 0.009 | 5 [2–12] | 0.393 | 75 [71–79] | 0.271 |
| AFB | 88 [74–95] | 50 [33–66] | 7 [4–12] | 76 [72–80] | ||||||||
| WLB versus AFB + WLB | 5 | 862 | 2,727 | WLB | 46 [20–73] | 0.062 | 91 [87–94] | <0.001 | 9 [3–24] | 0.413 | 89 [86–92] | <0.001 |
| AFB + WLB | 85 [54–97] | 71 [57–82] | 14 [4–51] | 81 [77–84] | ||||||||
| WLB versus NBI | 1 | 22 | 64 | WLB | 62 | – | 65 | – | – | – | – | – |
| NBI | 100 | 43 | – | – | ||||||||
WLB, white light bronchoscopy; AFB, autofluorescence bronchoscopy; AFB + WLB, AFB combined with WLB; NBI, narrow-band imaging; DOR, diagnostic odds ratio; AUC, area under the receiver-operating characteristic curve; CIS, carcinoma in situ.
Moreover, though AUC of WLB was non-inferior to that of AFB (75% versus 76%, P=0.271), even superior to the AFB + WLB (89% versus 81%, P<0.001), WLB still presented lower sensitivity and DOR for these high-graded lesions. Regarding NBI, we were limited to calculate the DOR and AUC based on the data from only one study (Table 3).
Meta-regression indicated there was no strong effect of study period and quality on the heterogeneity according to the data of single-arm synthesis with original criteria. Types of analysis (biopsy- versus patient-based) may be the source based on the I2 value, even if the P value of heterogeneity was not lower than 0.05 (Table 4).
Table 4. Meta-regression for source of heterogeneity.
| Source of heterogeneity | WLB | AFB | AFB + WLB | NBI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P* | I2 (95% CI) (%) | P* | I2 (95% CI) (%) | P* | I2 (95% CI) (%) | P* | I2 (95% CI) (%) | ||||
| Median year: past versus current | 0.57 | 0 [0–100] | 0.21 | 37 [0–100] | 0.85 | 0 [0–100] | 0.30 | 18 [0–100] | |||
| Quality: high versus moderate | 0.84 | 0 [0–100] | 0.10 | 56 [1–100] | 0.37 | 0 [0–100] | 0.19 | 39 [0–100] | |||
| Analysis: biopsy- versus patient-based | 0.10 | 57 [3–100] | 0.17 | 44 [0–100] | 0.09 | 58 [6–100] | 0.03 | 71 [37–100] | |||
*, P value was based on the joint model, considering the sensitivity and specificity together; P value <0.05, indicated the factors could be the source of heterogeneity during data synthesis. WLB, white light bronchoscopy; AFB, autofluorescence bronchoscopy; AFB + WLB, AFB combined with WLB; NBI, narrow-band imaging.
Discussion
In this systematic review and meta-analysis, we primarily performed single-arm synthesis of conventional WLB and advanced bronchoscopies for diagnosing early lung cancer in one article. Besides, we also conducted an exploratory direct comparison of these techniques. Based on original pathological criteria used in included studies, our findings indicated the sensitivity and overall diagnostic performance (DOR and AUC) of advanced bronchoscopies (AFB, AFB + WLB and NBI) were superior to those of WLB. Based on the pathological criteria from MOD to INV, higher sensitivity and DOR of AFB and AFB + WLB could still be found.
Findings of interest were that, based on the original pathological criteria from hyperplasia to INV, both over 80% sensitivity and specificity were indicated in NBI bronchoscopy in single-arm synthesis, as well as the significantly superior DOR and AUC of NBI were shown when compared with those of WLB (in direct comparison). This outcome could be a result of both the color change and characteristics of submucosa vessels seen with the use of NBI; such characteristics could help practitioners effectively recognize and distinguish malignant lesions from benign lesions (5,9).
However, based on the pathological criteria from MOD to INV, the specificity of NBI decreased to 43% (66). Regarding above findings, one consideration should be taken: the objective of the included studies was to investigate the performance of NBI for diagnosing lung cancer (cancerous lesions). Accordingly, among the lesions under positive criteria of pathology, the percentage of cancerous lesions was far larger than the percentage of precancerous lesions (63-68). Therefore, during statistical calculation, we assumed that the large percentage of cancerous lesions in positive lesions may account for the remarkably high sensitivity and specificity of NBI in our research. A question that whether this technique still present such high diagnostic performance for precancerous lesions in the central airway, still warranted more studies to answer.
Autofluorescence technique was reported with more advantages for early lung cancer than white light technique in three published meta-analyses (6-8). In our research, we analyzed their performance based on original pathological criteria in included studies and found the similar outcome—higher sensitivity, DOR and AUC of AFB and AFB + WLB than those of WLB. When we used the criteria from MOD to INV, we found difference: AFB and AFB + WLB did not present superior enough AUC to WLB. This finding has been not yet reported in previous meta-analyses (6-8). We assume, the comparable diagnostic accuracy of WLB for higher-degree lesions, especially INV, contributed the non-superiority of AFB and AFB + WLB regarding the AUC: in Sun et al. meta-analysis (6), the pooled sensitivity of WLB was 88.53% for invasive lesions, and 42.54% for intraepithelial neoplasm including moderate/severe dysplasia (SEV) and CIS. Regardless of the AUC of AFB and AFB + WLB versus WLB, their another overall index, DOR, paralleled with their sensitivity, were still higher than those of WLB.
Besides, in both single-arm synthesis and direct comparison, we also surprisingly found the specificity of AFB + WLB was over 70% for MOD to INV. Considering all diagnostic indexes (sensitivity, specificity, DOR and AUC) among AFB, AFB + WLB and WLB, the combination strategy of autofluorescence and white light techniques may be more useful to diagnose these range of lesions rather than their alone use. Based on this assumption, another meta-analysis comparing AFB and AFB + WLB directly is needed.
Considering the superior sensitivity and DOR of all advanced techniques we studied for different lesions (from hyperplasia to INV), comprehensive strategy could be further explored for patients. In current stage, for the low prevalence of invasive and high-grade lesions, we understand that using these techniques even in addition to computed tomography (CT) for screening lung cancer in high-risk population is still out of sufficient evidence (69). However, due to the potential property of pre-invasive lesions progressing as worse lesions, as well as the controversy that early intervention could be applied to treat these lesions, advanced bronchoscopies may be considered for the surveillance or follow-up when these lesions exist in airway (3,70,71). Furthermore, combined with genetic and epigenetic analysis, these advanced techniques may be useful to provide more evidence of diagnosis, chemoprevention and early endobronchial intervention for pre-invasive lesions in future studies (72-75).
Some limitations existed in our research. Firstly, there was no united standard of the inclusion criteria of study selection about which types or range of pathology should be regarded as the positive results. For example, one study regarded the range from metaplasia to SEV as the positive standard (17), but the range from MOD to CIS was used as the positive pathological result in another study (18). Accordingly, except the original criteria used in our included studies, we also conducted our analysis based on the specific range, from MOD to INV, which may hopefully address this issue and show the exact performance of these techniques when diagnosing. Secondly, the nature of our research limited us to consider other factors regarding the details of study procedure in all included researches for further analysis, such as single or independent diagnosis among bronchoscopists or pathologists, experience level of practitioners, random biopsy, etc. Moreover, our included studies of NBI were still lack of sufficiency. Considering its possibly high sensitivity and specificity, we expect more NBI studies not only for diagnosing lung cancer, but also precancerous lesions.
In conclusion, with remarkable sensitivity, we believe potential lesions of pre-cancerous and cancerous lesions could be covered by advanced techniques, especially precancerous lesions, almost invisible and easily missed by WLB. Combining strategy of AFB and WLB may contribute preferably rather than their alone use for detecting high-grade lesions from MOD to INV. NBI for airway precancerous lesions warrants further investigation.
Acknowledgements
We want to thank following contributors for providing assistance and suggestion in this work: Lawrence D. Grouse, Xiaoru Deng, Yi Zhang, Minzhang Guo, Xuewei Chen, Jingyi Chen, Jianfei Shen, Shengyi Zhong, Yang Liu, Zhihua Guo, Wei Wang, Shuben Li, Xusen Zou, Ying Chen, Huishan Wei, Tiansong Zhang, Wenzhao Zhong, Zhide Hu, Zhirui Zhou. Significantly, we want to give sincere thanks to all authors and patients of our included studies.
Funding: This work was supported by the Science and Technology Planning Project of Guangdong Province, China (grant numbers: 2007B031515017; 2008A030201024).
Table S1. Quality assessment of diagnostic accuracy studies-2 (QUADAS-2).
| Author | Risk of bias | Applicability concerns | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1Q1 | D1Q2 | D1Q3 | D1 | D2Q1 | D2Q2 | D2 | D3Q1 | D3Q2 | D3 | D4Q1 | D4Q2 | D4Q3 | D4 | D1 | D2 | D3 | ||
| Kurie 1998 | Y | Y | Y | L | Y | Y | L | Y | U | L | U | Y | N | U | L | L | L | |
| Moro-Sibilot 2002 | Y | Y | U | L | Y | Y | L | Y | Y | L | U | Y | N | U | L | L | L | |
| Herth 2003 | U | Y | U | U | Y | Y | L | Y | U | L | U | Y | N | U | L | L | L | |
| Lee 2007 | U | Y | Y | L | Y | Y | L | Y | U | L | U | Y | Y | L | L | L | L | |
| Moghissi 2008 | U | Y | Y | L | Y | Y | L | Y | U | L | U | Y | N | U | L | L | L | |
| Lee 2009 | U | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | Y | L | L | L | L | |
| Zaric 2009 | N | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | U | U | L | L | L | |
| Zaric 2010 | N | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | Y | L | L | L | L | |
| Lam 1992 | U | Y | U | U | Y | Y | L | Y | Y | L | U | Y | Y | L | L | L | L | |
| Lam 1993 | U | Y | U | U | Y | Y | L | Y | U | L | U | Y | N | U | L | L | L | |
| Lam 1994 | U | Y | U | U | Y | Y | L | Y | U | L | U | Y | Y | L | L | L | L | |
| Yokomise 1997 | U | Y | U | U | Y | Y | L | Y | Y | L | U | Y | Y | L | L | L | L | |
| Horvath 1999 | U | Y | Y | L | Y | Y | L | Y | U | L | U | Y | Y | L | L | L | L | |
| Kakihana 1999 | U | Y | U | U | Y | Y | L | Y | U | L | U | Y | Y | L | U | L | L | |
| Ikeda 1999 | U | Y | Y | L | Y | Y | L | Y | U | L | U | Y | Y | L | L | L | L | |
| Weigel 1999 | N | Y | U | U | Y | Y | L | Y | Y | L | U | Y | Y | L | U | L | L | |
| Weigel 2000 | U | Y | U | U | Y | Y | L | Y | U | L | U | Y | Y | L | U | L | L | |
| Shibuya 2001 | U | Y | U | U | Y | Y | L | Y | U | L | U | Y | N | U | U | L | L | |
| Furukawa 2000 | U | Y | U | U | Y | Y | L | Y | U | L | U | Y | Y | L | U | L | L | |
| Means-Markwell 2003 | U | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | N | U | L | L | L | |
| Lang 2005 | Y | Y | Y | L | Y | Y | L | Y | U | L | U | Y | Y | L | L | L | L | |
| Chhajed 2005 | Y | Y | Y | L | Y | Y | L | Y | U | L | U | Y | Y | L | L | L | L | |
| Chiyo 2005 | U | Y | U | U | Y | Y | L | Y | Y | L | U | Y | N | U | U | L | L | |
| Lam 2006 | Y | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | N | U | L | L | L | |
| Ikeda 2006 | Y | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | N | U | L | L | L | |
| Ueno 2007 | U | Y | Y | L | Y | Y | L | Y | U | L | U | Y | Y | L | L | L | L | |
| Nakanishi 2007 | U | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | Y | L | L | L | L | |
| Gabrecht 2007 | U | Y | Y | L | Y | Y | L | Y | U | L | U | Y | Y | L | L | L | L | |
| Li 2010 | U | Y | U | U | Y | Y | L | Y | U | L | U | Y | Y | L | U | L | L | |
| Ali 2011 | U | Y | Y | L | Y | Y | L | Y | U | L | U | Y | Y | L | L | L | L | |
| Cetýnkaya 2011 | U | Y | Y | L | Y | Y | L | Y | U | L | U | N | Y | U | L | L | L | |
| Venmans 1999 | U | Y | U | U | Y | U | L | Y | U | L | U | Y | Y | L | U | L | L | |
| Kusunoki 2000 | U | Y | U | U | Y | Y | L | Y | U | L | U | Y | Y | L | U | L | L | |
| Hirsch 2001 | U | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | Y | L | L | L | L | |
| Fuso 2005 | Y | Y | U | L | Y | Y | L | Y | Y | L | U | Y | Y | L | L | L | L | |
| Ernst 2005 | N | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | N | U | L | L | L | |
| Divisi 2010 | U | Y | U | U | Y | Y | L | Y | U | L | U | N | Y | U | U | L | L | |
| Lam 1998 | U | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | Y | L | L | L | L | |
| Vermylen 1999 | Y | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | N | U | L | L | L | |
| Häußinger 1999 | U | Y | U | U | Y | Y | L | Y | U | L | U | Y | Y | L | U | L | L | |
| Venmans 2000 | U | Y | U | U | Y | Y | L | Y | Y | L | U | Y | N | U | U | L | L | |
| Sato 2001 | U | Y | U | U | Y | Y | L | Y | U | L | U | Y | Y | L | U | L | L | |
| Häußinger 2005 | Y | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | N | U | L | L | L | |
| Hanibuchi 2007 | U | Y | U | U | Y | Y | L | Y | U | L | U | Y | Y | L | L | L | L | |
| Edell 2009 | U | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | N | U | L | L | L | |
| Xing 2005 | U | Y | Y | L | Y | U | L | Y | U | L | U | N | N | U | L | L | L | |
| Reinders 2009 | U | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | N | U | L | L | L | |
| Shibuya 2003 | U | Y | U | U | Y | Y | L | Y | U | L | U | Y | Y | L | L | L | L | |
| Bojan 2009 | N | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | U | U | L | L | L | |
| Zaric 2009 | N | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | Y | L | L | L | L | |
| Vincent 2007 | U | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | Y | L | L | L | L | |
| Nguyen 2013 | U | Y | Y | L | Y | Y | L | Y | U | L | U | Y | N | U | L | L | L | |
| Herth 2009 | U | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | N | U | L | L | L | |
Y, yes; N, no; U, unclear; L, low; H, high; risk of bias: D1, domain 1, patient selection; D2, domain 2, index test; D3, domain 3, reference standard; D4, domain 4, flow and timing; D1Q1, was a consecutive or random sample of patients enrolled? D1Q2, was a case-control design avoided? D1Q3, did the study avoid inappropriate exclusions? D2Q1, were the index test results interpreted without knowledge of the results of the reference standard? D2Q2, if a threshold was used, was it prespecified? D3Q1, is the reference standard likely to correctly classify the target condition? D3Q2, were the reference standard results interpreted without knowledge of the results of the index test? D4Q1, was there an appropriate interval between the index test and reference standard? D4Q2, did all patients receive the same reference standard? D4Q3, were all patients or biopsy specimens included in the analysis? Applicability concern: D1, domain 1, are there concerns that the included patients and setting do not match the review question? D2, domain 2, are there concerns that the index test, its conduct, or its interpretation differ from the review question? D3, domain 3, are there concerns that the target condition as defined by the reference standard does not match the question?
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Kassis ES, Vaporciyan AA, Swisher SG, et al. Application of the revised lung cancer staging system (IASLC Staging Project) to a cancer center population. J Thorac Cardiovasc Surg 2009;138:412-418.e1-2. [DOI] [PMC free article] [PubMed]
- 3.van Boerdonk RA, Smesseim I, Heideman DA, et al. Close Surveillance with Long-Term Follow-up of Subjects with Preinvasive Endobronchial Lesions. Am J Respir Crit Care Med 2015;192:1483-9. 10.1164/rccm.201504-0822OC [DOI] [PubMed] [Google Scholar]
- 4.Stanzel F. Fluorescent bronchoscopy: contribution for lung cancer screening? Lung Cancer 2004;45 Suppl 2:S29-37. 10.1016/j.lungcan.2004.07.995 [DOI] [PubMed] [Google Scholar]
- 5.East JE, Tan EK, Bergman JJ, et al. Meta-analysis: narrow band imaging for lesion characterization in the colon, oesophagus, duodenal ampulla and lung. Aliment Pharmacol Ther 2008;28:854-67. 10.1111/j.1365-2036.2008.03802.x [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Garfield DH, Lam B, et al. The value of autofluorescence bronchoscopy combined with white light bronchoscopy compared with white light alone in the diagnosis of intraepithelial neoplasia and invasive lung cancer: a meta-analysis. J Thorac Oncol 2011;6:1336-44. 10.1097/JTO.0b013e318220c984 [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Gao X, Tian Q, et al. A comparison of autofluorescence bronchoscopy and white light bronchoscopy in detection of lung cancer and preneoplastic lesions: a meta-analysis. Lung Cancer 2011;73:183-8. 10.1016/j.lungcan.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Wang Q, Feng J, et al. Comparison of autofluorescence imaging bronchoscopy and white light bronchoscopy for detection of lung cancers and precancerous lesions. Patient Prefer Adherence 2013;7:621-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iftikhar IH, Musani AI. Narrow-band imaging bronchoscopy in the detection of premalignant airway lesions: a meta-analysis of diagnostic test accuracy. Ther Adv Respir Dis 2015;9:207-16. 10.1177/1753465815589698 [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Reprint--preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther 2009;89:873-80. [PubMed] [Google Scholar]
- 11.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 12.Altman DG, Bland JM. Diagnostic tests. 1: Sensitivity and specificity. BMJ 1994;308:1552. 10.1136/bmj.308.6943.1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altman DG, Bland JM. Diagnostic tests 2: Predictive values. BMJ 1994;309:102. 10.1136/bmj.309.6947.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanders LK, East JE, Uitentuis SE, et al. Diagnostic performance of narrowed spectrum endoscopy, autofluorescence imaging, and confocal laser endomicroscopy for optical diagnosis of colonic polyps: a meta-analysis. Lancet Oncol 2013;14:1337-47. 10.1016/S1470-2045(13)70509-6 [DOI] [PubMed] [Google Scholar]
- 15.Leeflang MM. Systematic reviews and meta-analyses of diagnostic test accuracy. Clin Microbiol Infect 2014;20:105-13. 10.1111/1469-0691.12474 [DOI] [PubMed] [Google Scholar]
- 16.Kurie JM, Lee JS, Morice RC, et al. Autofluorescence bronchoscopy in the detection of squamous metaplasia and dysplasia in current and former smokers. J Natl Cancer Inst 1998;90:991-5. 10.1093/jnci/90.13.991 [DOI] [PubMed] [Google Scholar]
- 17.Moro-Sibilot D, Jeanmart M, Lantuejoul S, et al. Cigarette smoking, preinvasive bronchial lesions, and autofluorescence bronchoscopy. Chest 2002;122:1902-8. 10.1378/chest.122.6.1902 [DOI] [PubMed] [Google Scholar]
- 18.Herth FJ, Becker HD, LoCicero J, III, et al. Endobronchial ultrasound improves classification of suspicious lesions detected by autofluorescence bronchoscopy. J Bronchology Interv Pulmonol 2003;10:249-52. [Google Scholar]
- 19.Lee P, Brokx HA, Postmus PE, et al. Dual digital video-autofluorescence imaging for detection of pre-neoplastic lesions. Lung Cancer 2007;58:44-9. 10.1016/j.lungcan.2007.04.009 [DOI] [PubMed] [Google Scholar]
- 20.Moghissi K, Dixon K, Stringer M, et al. Autofluorescence bronchoscopy to detect bronchial epithelial changes associated with cigarette smoking among asymptomatic volunteers: a single center prospective pilot study. J Bronchology Interv Pulmonol 2008;15:21-6. [Google Scholar]
- 21.Lee P, van den Berg RM, Lam S, et al. Color fluorescence ratio for detection of bronchial dysplasia and carcinoma in situ. Clin Cancer Res 2009;15:4700-5. 10.1158/1078-0432.CCR-08-1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaric B, Canak V, Stojanovic G, et al. Autofluorescence videobronchoscopy (AFI) for the assessment of tumor extension in lung cancer. Technol Cancer Res Treat 2009;8:79-84. 10.1177/153303460900800110 [DOI] [PubMed] [Google Scholar]
- 23.Zaric B, Becker HD, Perin B, et al. Autofluorescence imaging videobronchoscopy improves assessment of tumor margins and affects therapeutic strategy in central lung cancer. Jpn J Clin Oncol 2010;40:139-45. 10.1093/jjco/hyp135 [DOI] [PubMed] [Google Scholar]
- 24.Lam S, Hung JY, Kennedy SM, et al. Detection of Dysplasia and Carcinoma In Situ by Ratio Fluorometry1-3. Am Rev Respir Dis 1992;146:1458-61. 10.1164/ajrccm/146.6.1458 [DOI] [PubMed] [Google Scholar]
- 25.Lam S, MacAulay C, Hung J, et al. Detection of dysplasia and carcinoma in situ with a lung imaging fluorescence endoscope device. J Thorac Cardiovasc Surg 1993;105:1035-40. [PubMed] [Google Scholar]
- 26.Lam S, Macaulay C, Leriche JC, et al. Early localization of bronchogenic carcinoma. Diagn Ther Endosc 1994;1:75-8. 10.1155/DTE.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokomise H, Yanagihara K, Fukuse T, et al. Clinical Experience with Lung-Imaging Fluorescence Endoscope (LIFE) in Patients with Lung Cancer. J Bronchology Interv Pulmonol 1997;4:205-8. [Google Scholar]
- 28.Horvath T, Horvathova M, Salajka F, et al. Detection of Bronchial Neoplasia in Uranium Miners by Autofluorescence Endoscopy (SAFE-1000). Diagn Ther Endosc 1999;5:91-8. 10.1155/DTE.5.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakihana M, Kyong IK, Okunaka T, et al. Early detection of bronchial lesions using system of autofluorescence endoscopy (SAFE) 1000. Diagn Ther Endosc 1999;5:99-104. 10.1155/DTE.5.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeda N, Honda H, Katsumi T, et al. Early detection of bronchial lesions using lung imaging fluorescence endoscope. Diagn Ther Endosc 1999;5:85-90. 10.1155/DTE.5.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weigel TL, Kosco PJ, Dacic S, et al. Fluorescence bronchoscopic surveillance in patients with a history of non-small cell lung cancer. Diagn Ther Endosc 1999;6:1-7. 10.1155/DTE.6.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weigel TL, Yousem S, Dacic S, et al. Fluorescence bronchoscopic surveillance after curative surgical resection for non-small-cell lung cancer. Ann Surg Oncol 2000;7:176-80. 10.1007/BF02523650 [DOI] [PubMed] [Google Scholar]
- 33.Shibuya K, Fujisawa T, Hoshino H, et al. Fluorescence bronchoscopy in the detection of preinvasive bronchial lesions in patients with sputum cytology suspicious or positive for malignancy. Lung Cancer 2001;32:19-25. 10.1016/S0169-5002(00)00200-2 [DOI] [PubMed] [Google Scholar]
- 34.Furukawa K, Kakihana M, Ikeda N, et al. Fluorescence bronchoscopy in the early detection of premalignant and malignant lesions. J Jpn Soc Respir Endosc 2000;22:629-35. [Google Scholar]
- 35.Means-Markwell M, Linnoila RI, Williams J, et al. Prospective study of the airways and pulmonary parenchyma of patients at risk for a second lung cancer. Clin Cancer Res 2003;9:5915-21. [PubMed] [Google Scholar]
- 36.Lang SM, Ebelt K, Hautmann H, et al. Detection of cancerous endobronchial lesions by autofluorescence bronchoscopy combined with mutation analysis of p53. Eur J Med Res 2005;10:273-7. [PubMed] [Google Scholar]
- 37.Chhajed PN, Shibuya K, Hoshino H, et al. A comparison of video and autofluorescence bronchoscopy in patients at high risk of lung cancer. Eur Respir J 2005;25:951-5. 10.1183/09031936.05.00012504 [DOI] [PubMed] [Google Scholar]
- 38.Chiyo M, Shibuya K, Hoshino H, et al. Effective detection of bronchial preinvasive lesions by a new autofluorescence imaging bronchovideoscope system. Lung Cancer 2005;48:307-13. 10.1016/j.lungcan.2004.11.023 [DOI] [PubMed] [Google Scholar]
- 39.Lam B, Wong M, Fung S, et al. The clinical value of autofluorescence bronchoscopy for the diagnosis of lung cancer. Eur Respir J 2006;28:915-9. 10.1183/09031936.06.00131405 [DOI] [PubMed] [Google Scholar]
- 40.Ikeda N, Honda H, Hayashi A, et al. Early detection of bronchial lesions using newly developed videoendoscopy-based autofluorescence bronchoscopy. Lung Cancer 2006;52:21-7. 10.1016/j.lungcan.2005.11.009 [DOI] [PubMed] [Google Scholar]
- 41.Ueno K, Kusunoki Y, Imamura F, et al. Clinical experience with autofluorescence imaging system in patients with lung cancers and precancerous lesions. Respiration 2007;74:304-8. 10.1159/000093233 [DOI] [PubMed] [Google Scholar]
- 42.Nakanishi K, Ohsaki Y, Kurihara M, et al. Color auto-fluorescence from cancer lesions: improved detection of central type lung cancer. Lung Cancer 2007;58:214-9. 10.1016/j.lungcan.2007.06.009 [DOI] [PubMed] [Google Scholar]
- 43.Gabrecht T, Radu A, Zellweger M, et al. Detection of early bronchial cancer by autofluorescence: results in patients with H&N cancer. European Conference on Biomedical Optics 2007;6628:6628C1-8. [Google Scholar]
- 44.Li Y, Li X, Sui XZ, et al. Comparison of the autofluorescence bronchoscope and the white light bronchoscope in airway examination. Chin J Cancer 2010;29:1018-22. 10.5732/cjc.010.10298 [DOI] [PubMed] [Google Scholar]
- 45.Ali AH, Takizawa H, Kondo K, et al. Follow-up using fluorescence bronchoscopy for the patients with photodynamic therapy treated early lung cancer. J Med Invest 2011;58:46-55. 10.2152/jmi.58.46 [DOI] [PubMed] [Google Scholar]
- 46.Cetınkaya E, Veyseller B, Yildirim YS, et al. Value of autofluorescence bronchoscopy in patients with laryngeal cancer. J Laryngol Otol 2011;125:181-7. 10.1017/S002221511000229X [DOI] [PubMed] [Google Scholar]
- 47.Venmans BJ, Van Boxem TJ, Smit EF, et al. Results of two years expenience with fluorescence bronchoscopy in detection of preinvasive bronchial neoplasia. Diagn Ther Endosc 1999;5:77-84. 10.1155/DTE.5.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kusunoki Y, Imamura F, Uda H, et al. Early detection of lung cancer with laser-induced fluorescence endoscopy and spectrofluorometry. Chest 2000;118:1776-82. 10.1378/chest.118.6.1776 [DOI] [PubMed] [Google Scholar]
- 49.Hirsch FR, Prindiville SA, Miller YE, et al. Fluorescence versus white-light bronchoscopy for detection of preneoplastic lesions: a randomized study. J Natl Cancer Inst 2001;93:1385-91. 10.1093/jnci/93.18.1385 [DOI] [PubMed] [Google Scholar]
- 50.Fuso L, Pagliari G, Boniello V, et al. Autofluorescence bronchoscopy to identify pre-cancerous bronchial lesions. Monaldi Arch Chest Dis 2005;63:124-8. 10.4081/monaldi.2005.628 [DOI] [PubMed] [Google Scholar]
- 51.Ernst A, Simoff MJ, Mathur PN, et al. D-light autofluorescence in the detection of premalignant airway changes: a multicenter trial. J Bronchology Interv Pulmonol 2005;12:133-8. [Google Scholar]
- 52.Divisi D, Di Tommaso S, De Vico A, et al. Early diagnosis of lung cancer using a SAFE-3000 autofluorescence bronchoscopy. Interact Cardiovasc Thorac Surg 2010;11:740-4. 10.1510/icvts.2010.242123 [DOI] [PubMed] [Google Scholar]
- 53.Lam S, Kennedy T, Unger M, et al. Localization of bronchial intraepithelial neoplastic lesions by fluorescence bronchoscopy. Chest 1998;113:696-702. 10.1378/chest.113.3.696 [DOI] [PubMed] [Google Scholar]
- 54.Vermylen P, Pierard P, Roufosse C, et al. Detection of bronchial preneoplastic lesions and early lung cancer with fluorescence bronchoscopy: a study about its ambulatory feasibility under local anaesthesis. Lung Cancer 1999;25:161-8. 10.1016/S0169-5002(99)00058-6 [DOI] [PubMed] [Google Scholar]
- 55.Häußinger K, Stanzel F, Huber RM, et al. Autofluorescence Detection of Bronchial Tumors With the D-Light/AF. Diagn Ther Endosc 1999;5:105-12. 10.1155/DTE.5.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venmans BJ, van der Linden HC, Elbers HR, et al. Observer Variability in Histopathologic Reporting of Bronchial Biopsy Specimens: Influence on the Results of Autofluorescence Bronchoscopy in Detection of Preinvasive Bronchial Neoplasia. J Bronchology Interv Pulmonol 2000;7:210-4. [Google Scholar]
- 57.Sato M, Sakurada A, Sagawa M, et al. Diagnostic results before and after introduction of autofluorescence bronchoscopy in patients suspected of having lung cancer detected by sputum cytology in lung cancer mass screening. Lung Cancer 2001;32:247-53. 10.1016/S0169-5002(00)00229-4 [DOI] [PubMed] [Google Scholar]
- 58.Häussinger K, Becker H, Stanzel F, et al. Autofluorescence bronchoscopy with white light bronchoscopy compared with white light bronchoscopy alone for the detection of precancerous lesions: a European randomised controlled multicentre trial. Thorax 2005;60:496-503. 10.1136/thx.2005.041475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanibuchi M, Yano S, Nishioka Y, et al. Autofluorescence bronchoscopy, a novel modality for the early detection of bronchial premalignant and malignant lesions. J Med Invest 2007;54:261-6. 10.2152/jmi.54.261 [DOI] [PubMed] [Google Scholar]
- 60.Edell E, Lam S, Pass H, et al. Detection and localization of intraepithelial neoplasia and invasive carcinoma using fluorescence-reflectance bronchoscopy: an international, multicenter clinical trial. J Thorac Oncol 2009;4:49-54. 10.1097/JTO.0b013e3181914506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xing S, Khanavkar B, Nakhosteen JA, et al. Predictive value of image cytometry for diagnosis of lung cancer in heavy smokers. Eur Respir J 2005;25:956-63. 10.1183/09031936.05.00118903 [DOI] [PubMed] [Google Scholar]
- 62.Reinders D, Snead D, Dhillon P, et al. Endobronchial cancer detection using an integrated bronchoscopy system for simultaneous imaging and noncontact spectral measurement. J Bronchology Interv Pulmonol 2009;16:158-67. 10.1097/LBR.0b013e3181ace2d5 [DOI] [PubMed] [Google Scholar]
- 63.Shibuya K, Hoshino H, Chiyo M, et al. High magnification bronchovideoscopy combined with narrow band imaging could detect capillary loops of angiogenic squamous dysplasia in heavy smokers at high risk for lung cancer. Thorax 2003;58:989-95. 10.1136/thorax.58.11.989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bojan Z, Branislav P, Aleksandra J, et al. Influence of narrow band imaging (NBI) videobronchoscopy on the assessment of central lung cancer extension and therapeutic decision. Cancer Invest 2009;27:918-23. 10.3109/07357900902918445 [DOI] [PubMed] [Google Scholar]
- 65.Zaric B, Becker HD, Perin B, et al. Narrow band imaging videobronchoscopy improves assessment of lung cancer extension and influences therapeutic strategy. Jpn J Clin Oncol 2009;39:657-63. 10.1093/jjco/hyp083 [DOI] [PubMed] [Google Scholar]
- 66.Vincent BD, Fraig M, Silvestri GA. A pilot study of narrow-band imaging compared to white light bronchoscopy for evaluation of normal airways and premalignant and malignant airways disease. Chest 2007;131:1794-9. 10.1378/chest.06-2794 [DOI] [PubMed] [Google Scholar]
- 67.Nguyen P, Bashirzadeh F, Hodge R, et al. High specificity of combined narrow band imaging and autofluorescence mucosal assessment of patients with head and neck cancer. Head Neck 2013;35:619-25. 10.1002/hed.22999 [DOI] [PubMed] [Google Scholar]
- 68.Herth FJ, Eberhardt R, Anantham D, et al. Narrow-band imaging bronchoscopy increases the specificity of bronchoscopic early lung cancer detection. J Thorac Oncol 2009;4:1060-5. 10.1097/JTO.0b013e3181b24100 [DOI] [PubMed] [Google Scholar]
- 69.Tremblay A, Taghizadeh N, McWilliams AM, et al. Low Prevalence of High-Grade Lesions Detected With Autofluorescence Bronchoscopy in the Setting of Lung Cancer Screening in the Pan-Canadian Lung Cancer Screening Study. Chest 2016;150:1015-22. 10.1016/j.chest.2016.04.019 [DOI] [PubMed] [Google Scholar]
- 70.Breuer RH, Pasic A, Smit EF, et al. The natural course of preneoplastic lesions in bronchial epithelium. Clin Cancer Res 2005;11:537-43. [PubMed] [Google Scholar]
- 71.Ishizumi T, McWilliams A, MacAulay C, et al. Natural history of bronchial preinvasive lesions. Cancer Metastasis Rev 2010;29:5-14. 10.1007/s10555-010-9214-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Boerdonk RA, Sutedja TG, Snijders PJ, et al. DNA copy number alterations in endobronchial squamous metaplastic lesions predict lung cancer. Am J Respir Crit Care Med 2011;184:948-56. 10.1164/rccm.201102-0218OC [DOI] [PubMed] [Google Scholar]
- 73.Pipinikas CP, Kiropoulos TS, Teixeira VH, et al. Cell migration leads to spatially distinct but clonally related airway cancer precursors. Thorax 2014;69:548-57. 10.1136/thoraxjnl-2013-204198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silvestri GA, Vachani A, Whitney D, et al. A bronchial genomic classifier for the diagnostic evaluation of lung cancer. N Engl J Med 2015;373:243-51. 10.1056/NEJMoa1504601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lam S, Szabo E. Preinvasive Endobronchial Lesions: Lung Cancer Precursors and Risk Markers? Am J Respir Crit Care Med 2015;192:1411-3. 10.1164/rccm.201508-1668ED [DOI] [PMC free article] [PubMed] [Google Scholar]