Abstract
This study intends to investigate the correlations of miR-124a and miR-30d with clinicopathological features of breast cancer (BC) patients with type 2 diabetes mellitus (T2DM). A total of 72 BC patients with T2DM (diabetic group) and 144 BC patients without T2DM (non-diabetic group) were enrolled in this study. Blood glucose was detected by glucose oxidase methods. Glycosylated hemoglobin (HbA1c) was measured by high performance liquid chromatography. Fasting insulin (FIns) was measured by chemiluminescent microparticle immunoassay. Automatic biochemical analyzer was used to detect triglyceride, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C). Estradiol (E2) was detected by radioimmunoassay. Homeostasis model assessment was applied to assess the insulin resistance (HOMA-IR) and β-cell insulin secretion (HOMA-IS). The expressions of miR124a and miR-30d were measured by quantitative real-time polymerase chain reaction (qRT-PCR). There were significant differences in age, the ratio of menopause, body mass index (BMI), HDL-C, TC, 2-h plasma glucose (2hPG), FIns, HbA1c, HOMA-IS and HOMA-IR between the diabetic and non-diabetic groups. The diabetic group had higher incidence of lymph node metastasis than non-diabetic group. The miR-124a expression was down-regulated while the miR-30d expression was up-regulated in BC patients with T2DM. The correlation analysis showed that miR-124a expression was positively correlated with HDL-C, while it was negatively correlated with age, HbA1c, LDL-C and E2. However, the miR-30d expression was negatively correlated with HDL-C but positively correlated with age, HbA1c, LDL-C and E2. In conclusion, miR-124a and miR-30d may be correlated with clinicopathological features of BC patients with T2DM. The miR-124a and miR-30d could serve as novel biomarkers for early diagnosis of BC in patients with T2DM.
Keywords: MicroRNA-124a, MicroRNA-30d, Breast cancer, Type 2 diabetes mellitus, Clinicopathological features
Background
Breast cancer (BC) is one of the most common malignant neoplasms for females with 1.4 million new diagnoses a year worldwide, and is one of the leading causes of cancer-related death (Ban and Godellas 2014). Diabetes mellitus (DM), commonly referred to as diabetes, is a group of metabolic diseases with high blood sugar levels over a prolonged period, and more than 95% of all DM cases is type 2 diabetes mellitus (T2DM) (American Diabetes 2013, 2014). It is reported that approximately 7% of the people worldwide in the age between 20 and 79 years is estimated to have DM in 2010 and the number is expected to rise by more than 50% in the next 20 years (Ginter and Simko 2012). Further, studies focused on the DM have demonstrated that DM was implicated in various cancers development, including pancreas cancer, colon cancer, liver cancer, esophagus cancer, endometrial cancer and BC (Chang et al. 2012; Pan et al. 2012; Shikata et al. 2013; Wang et al. 2012). The relative risk for mortality of BC patients has been reported to be twofold greater in BC patients with DM than in those without DM (Liao et al. 2011), and the epidemiologic evidences showed that DM patients have a significant higher risk of BC and is closely associated with a poor prognosis of BC patients (Luo et al. 2014; Oppong et al. 2014). The BC patients with T2DM had been reported to be more likely to associate with a higher incidence of lymph nodes and were at an advanced tumor stage, indicating a shorter survival time (He et al. 2015).
MicroRNAs (miRs) have been reported to be implicated in various malignancies and involved in a variety of biological processes, including cell proliferation, differentiation, apoptosis, and metastasis (Di Leva et al. 2014; Farazi et al. 2013). Acting as an abundant miR in the central neuron system, miR-124a has been reported to be linked to the progression of various tumors and it may act as an important regulator of the transcriptional protein network in beta-cells responsible for regulating intracellular signaling (Baroukh et al. 2007; Chen et al. 2013). Overexpression of miR-124a may inhibit the glucose-stimulated insulin secretion and the altered expression of miR-124a may lead to beta-cell dysfunction in T2DM patients (Sebastiani et al. 2015). It has been reported that the methylation of miR-124a in adjacent normal mucosa may be correlated with the microsatellite instability of colorectal cancer (Deng et al. 2011). Further, miR-30d regulates various physiological processes in normal tissues or cancer cells, including development, metastasis, apoptosis, senescence, proliferation and differentiation (Bridge et al. 2012; Zhao et al. 2012). MiR-30d may be acted as a novel oncogene that may be implicated in the development of tumors and homeostasis, and may be served as a potential useful biomarker or drug target in human malignancies (Yang et al. 2013). MiR-30d also plays a key role in activating glucose-induced insulin gene transcription and in avoiding beta-cell functions impaired by pro-inflammatory cytokines, which may act as a potential target for diabetes intervention (Zhao et al. 2012).
Indeed, both miR-124a and miR-30d may participate in the development of BC and T2DM occurrence. In the present study, we investigated the expressions of miR-124a and miR-30d in BC patients with T2DM, and analyzed correlations of miR-124a and miR-30d with clinicopathological features of BC patients with T2DM.
Methods
Study subjects
Between January 2012 and January 2015, a total of 72 patients diagnosed as BC with T2DM at Linyi People’s Hospital were enrolled as diabetic group in this study. According to the principle of 1:2, 144 patients diagnosed as BC without T2DM were randomly recruited as non-diabetic group using the random number table. The matching principle was the date of hospital visiting ±1 month. All patients were confirmed as BC patients through the paraffin slide biopsy via the Department of pathology. The diagnosis of T2DM was as following: (1) patients were inquired of medical history (diagnosed as T2DM by secondary-and tertiary-level hospitals) by physician preoperative, medication history and the condition of blood sugar monitoring; (2) fasting blood glucose (FPG) ≥7.0 mmol/L at admission or random blood glucose levels ≥11.11 mmol/L, or the 2-h plasma glucose (2hPG) from an oral glucose tolerance test (OGTT) ≥11.1 mmol/L. Inclusion criteria: all patients had a complete clinical data, including age, body mass index (BMI), family history, past medical history, menopausal status, tumor size, axillary lymph nodes, etc. Exclusion criteria: (1) BC patients with type 1 diabetes mellitus or secondary diabetes mellitus; (2) male BC patients; (3) patients with bilateral BC; (4) patients with carcinoma in situ of breast or stage IV BC patients; (5) BC patients who had received neoadjuvant chemotherapy or estrogen therapy; (6) patients with incomplete clinical data; and (7) patients with nonstandard anticancer therapy. Among the 72 BC patients with T2DM, most of them were treated with oral hypoglycemic agents, but the patients with poor control of glucose level before operation were treated with insulin therapy after physician consultation from Department of endocrinology. All the patients enrolled in the study were underwent unilateral modified radical mastectomy, and received 6 cycles of TAC chemotherapy (Taxotere + Adriamycin + cyclophosphamide) postoperatively. The BC patients with lymph nodes were received postoperative radiotherapy, and the patients with hormone-receptor-positive BC who received endocrine therapy. All patients were appropriately informed about this study and signed their informed consents forms. This study was conducted with the approval of the ethics committee of Linyi People’s Hospital, and the ethical approval for this study conformed to the standards of the Declaration of Helsinki (Pn 2014).
The medical records were reviewed, including the admission number, name, age, BMI, menopausal status, history of diabetes, family history of BC, past medical history, diameter of tumor, pathological types, metastasis of axillary lymph nodes, tumor stage, histological grade, immunohistochemical markers (estrogen receptor: ER; progesterone receptor: PR; human epithelial growth factor receptor 2: Her-2, P-glyprotein: P-gp; topoisomerase II: Topo-II; glatocnine-S-tranferase-π: GST-π), surgical procedures, operation time, chemotherapy regimens and course of chemotherapy. All patients were followed up after surgery. The tumor node metastasis (TNM) stages were classified in accordance with the grading standard published by the American Joint Committee on Cancer (AJCC) or Union for International Cancer Control (UICC) (Singletary et al. 2003).
Biochemical parameters
Blood glucose was determined by glucose oxidase method using a Roche glucometer (Accu-Chek Active, Roche Ltd., Germany). Glycosylated hemoglobin (HbA1c) was measured by high performance liquid chromatography (ADAMSTMA1c HA-8160, Japan). Fasting insulin (FIns) was detected by chemiluminescent microparticle immunoassay (CMIA; Abbott i2000SR, USA). Triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were detected by automatic biochemical analyzer (Roche Ltd., Germany). Homeostasis model assessment was applied to assess insulin resistance and β-cell insulin secretion, i.e. HOMA-IR = (FPG × FIns)/22.5. HOMA of β-cell insulin secretion (HOMA-IS) = 20 × FIns/(FPG − 3.5).
The last menstrual periods of patients were recorded, and the concentration of E2 in the follicular phase at 2–4 days after menstruation was detected. The E2 level in the blood serum was measured by radioimmunoassay (radio-immunity kits, Depp Biological Technology and Medical Products Co. Ltd, Tianjin, China), with intra-assay coefficient of variance (CV) 7.96% and inter-assay CV 9.22%.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Blood sample from the each patient after 12 h fasting was collected in the next morning, and was centrifuged following the instructions of RNA extraction kit. Total RNA was extracted by a miRNeasy Mini Kit (Qiagen Company, Hilden, Germany). RNA samples (5 μL) were diluted 20 times in RNA-free ultrapure water. The concentration and quality of RNA were determined by the ultraviolet absorbance at 260 and 280 nm (optical density, OD; OD260/OD280 ratio) using an ultraviolet spectrophotometer. The OD260/OD280 ratio between 1.7 and 2.1 indicated that the RNA had high purity, which could meet the requirements of further research processes. The cDNA template was generated by reverse transcription in a PCR amplifier. The qRT-PCR was conducted by ABI 7500 quantitative PCR System (Life Technologies, USA). The reaction condition was 40 cycles of denaturation at 95 °C for 10 min, denaturation at 95 °C for 10 s, annealing at 60 °C for 20 s and extension at 72 °C for 34 s. The primers were synthesized by Sangon Biotech (Shanghai, China), as illustrated in Table 1. U6 snRNA was used as an internal control. The cycle number at threshold (Ct value) was used to calculate the relative expressions of miR124a and miR30d. The results were presented as fold change, and calculated using the 2−ΔΔCT method (Livak and Schmittgen 2001), with the formula as: ΔΔCT = ΔCtdiabetic group –ΔCtnon-diabetic group, ΔCt = CtmiR – CtU6. The experiments were totally repeated for 3 times.
Table 1.
The primer sequences for qRT-PCR
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| U6 | GCTTCGGCAGCACATATACTAAAAT | CGCTTCACGAATTTGCGTGTCAT |
| miR-124a | UUAAGGCACGCGGUGAAUGCCA | CTTAAGGCACGCGGTGAATGCCA |
| miR-30d | UGUAAACAUCCCCGACUGGAAG | TGTAAACATCCCCGACTGGAAGA |
qRT-PCR quantitative real-time polymerase chain reaction
Statistical analysis
SPSS19.0 was used to conduct the statistical analysis. Measurement data were expressed as mean ± standard deviation (SD). Variance homogeneity test was used before analysis, and One-Way ANOVA analysis was used for multiple group comparisons. The least significant difference (LSD)-t test or Chi square test was used in pairwise comparison of averages among groups. The relationships between the expressions of miR-124a and miR-30d and clinicopathological features and biochemical parameters were analyzed by Pearson correlation analysis and linear regression analysis. P < 0.05 showed statistically significant.
Results
Comparisons of clinical features and biochemical parameters between the diabetic group and non-diabetic group
The mean age of patients in the diabetic group was 52.72 ± 6.23 years, and the mean BMI was 24.68 ± 4.74 kg/m2. In the diabetic group, 33.61% cases were premenopausal patients and 76.39% cases were postmenopausal patients. The mean age of patients in the non-diabetic group was 50.08 ± 4.76 years and the mean BMI was 23.21 ± 3.25 kg/m2. Of the 144 patients in the non-diabetic group, 44.44% cases were premenopausal patients and 55.56% cases were postmenopausal patients. There were significant differences on age, the ratio of menopause and BMI between the two groups (age: P < 0.001; ratio of menopause; P = 0.003; BMI: P = 0.008). Further, significant differences were observed in HDL-C, LDL-C, TC, FPG, 2hPG, FIns, HbA1c, HOMA-IS, HOMA-IR and E2 between diabetic group and non-diabetic group (all P < 0.05). The family history of BC, underlying diseases, systolic blood pressure (SBP), diastolic blood pressure (DBP) or TG had no significant differences between the two groups (all P > 0.05), as illustrated in Table 2.
Table 2.
Comparisons of clinical features and biochemical parameters between diabetic group and non-diabetic group
| Diabetic group (n = 72) | Non-diabetic group (n = 144) | t/χ2 | P value | |
|---|---|---|---|---|
| Age (years) | 52.72 ± 6.23 | 50.08 ± 4.76 | 3.113 | <0.001 |
| Family history of BC | 5.56% (4) | 4.17% (6) | 0.458 | 0.647 |
| Underlying diseases | 19.44% (14) | 20.83% (30) | 0.057 | 0.811 |
| Ratio of menopause | 76.39% (55) | 55.56% (80) | 8.889 | 0.003 |
| BMI (Kg/m2) | 24.68 ± 4.74 | 23.21 ± 3.25 | 2.673 | 0.008 |
| SBP (mmHg) | 124.68 ± 10.35 | 125.36 ± 11.23 | 0.430 | 0.667 |
| DBP (mmHg) | 78.36 ± 7.93 | 80.57 ± 8.54 | 1.835 | 0.068 |
| HDL-C (mmol/L) | 1.21 ± 0.32 | 1.31 ± 0.36 | 1.995 | 0.047 |
| LDL-C (mmol/L) | 3.85 ± 0.74 | 3.12 ± 0.65 | 7.425 | <0.001 |
| TG (mmol/L) | 1.32 ± 0.35 | 1.41 ± 0.41 | 1.594 | 0.112 |
| TC (mmol/L) | 4.45 ± 0.87 | 3.64 ± 0.68 | 7.498 | <0.0001 |
| FPG (mmol/L) | 8.32 ± 0.73 | 5.27 ± 0.43 | 38.56 | <0.001 |
| 2hPG (mmol/L) | 14.68 ± 1.02 | 5.58 ± 0.47 | 89.810 | <0.001 |
| HbA1c (%) | 10.57 ± 2.11 | 5.64 ± 1.57 | 19.320 | <0.001 |
| FIns (uU/mL) | 10.14 ± 3.25 | 5.33 ± 1.87 | 6.954 | <0.001 |
| HOMA-IS | 42.84 ± 14.53 | 60.50 ± 16.29 | 7.779 | <0.001 |
| HOMA-IR | 3.75 ± 1.25 | 1.27 ± 0.50 | 20.750 | <0.001 |
| E2 (pg/mL) | 50.20 ± 11.40 | 23.66 ± 8.63 | 19.080 | <0.001 |
Comparisons on family history of BC, underlying diseases and the ratio of menopause between the two groups were measured by χ2 test; BMI, weight (kg)/height (m2); Normal BMI, 18.5–22.9 kg/m2
Diabetic group, breast cancer patients with type 2 diabetes mellitus; Non-diabetic group, breast cancer patients without type 2 diabetes mellitus
BC breast cancer, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, TG triglyceride, TC total cholesterol, FPG fasting blood glucose, 2hPG 2-hour postprandial blood glucose, HbA1c glycosylated hemoglobin, FIns fasting insulin, HOMA-IR homeostasis model assessment of insulin resistance, HOMA-IS HOMA of β-cell insulin secretion, E 2 estradiol
Comparisons of pathological features between the diabetic group and non-diabetic group
The comparisons of pathological features indicated that diabetic group had higher incidence of lymph node metastasis than non-diabetic group (P < 0.001). However, there were no significant differences in diameter of tumor, ratio of axillary lymph nodes, pathological types, tumor stage, histological grade, ER, PR, Her-2, P-gp, Topo-II and Gst-π (all P > 0.05) (Table 3).
Table 3.
Comparisons of pathological characteristics between diabetic group and non-diabetic group
| Characteristic | Diabetic group (n = 72) [%] | Non-diabetic group (n = 144) [%] | χ2 | P value |
|---|---|---|---|---|
| T stage | ||||
| T1 | 16 (22.2) | 36 (25.0) | 0.809 | 0.667 |
| T2 | 35 (48.6) | 74 (51.4) | ||
| T3 | 21 (29.2) | 34 (23.6) | ||
| Axillary lymph nodes | ||||
| Positive | 45 (62.5) | 75 (52.1) | 2.109 | 0.146 |
| Negative | 27 (37.5) | 69 (47.9) | ||
| Pathological types | ||||
| Invasive ductal carcinoma | 66 (91.7) | 136 (94.4) | 0.890 | 0.828 |
| Invasive lobular carcinoma | 3 (4.2) | 3 (2.1) | ||
| Invasive papilloma | 2 (2.8) | 3 (2.1) | ||
| Mucinous adenocarcinoma | 1 (1.4) | 2 (1.4) | ||
| Tumor stage | ||||
| I | 4 (5.6) | 19 (13.2) | 2.966 | 0.227 |
| II | 27 (37.5) | 51 (35.4) | ||
| III | 41 (56.9) | 74 (51.4) | ||
| Histological grade | ||||
| WHO I | 5 (6.9) | 9 (6.2) | 2.099 | 0.350 |
| WHO II | 42 (58.3) | 98 (68.1) | ||
| WHO III | 25 (34.7) | 37 (25.7) | ||
| Lymph node metastasis | ||||
| Positive | 45 (62.5) | 55 (38.2) | 11.410 | <0.001 |
| Negative | 27 (37.5) | 89 (61.8) | ||
| ER | ||||
| Positive | 42 (58.3) | 88 (61.1) | 0.155 | 0.694 |
| Negative | 30 (41.7) | 56 (38.9) | ||
| PR | ||||
| Positive | 40 (55.6) | 91 (63.2) | 1.174 | 0.279 |
| Negative | 32 (44.4) | 53 (36.8) | ||
| Her-2 | ||||
| Positive | 25 (34.7) | 66 (458) | 2.431 | 0.119 |
| Negative | 47 (65.3) | 78 (54.2) | ||
| P-gp | ||||
| Positive | 60 (83.3) | 121 (84.0) | 0.017 | 0.896 |
| Negative | 12 (16.7) | 23 (16.0) | ||
| Topo-II | ||||
| Positive | 64 (88.9) | 132 (91.7) | 0.441 | 0.507 |
| Negative | 8 (11.1) | 12 (8.3) | ||
| Gst-π | ||||
| Positive | 39 (54.2) | 70 (48.6) | 0.593 | 0.441 |
| Negative | 33 (45.8) | 74 (51.4) | ||
Diabetic group, breast cancer patients with type 2 diabetes mellitus; non-Diabetic group, breast cancer patients without type 2 diabetes mellitus
WHO World Health Organization, ER estrogen receptor, PR progesterone receptor, Her-2 human epithelial growth factor receptor 2, P-gp P-glyprotein, Topo-II topoisomerase II, Gst-π glatocnine-S-tranferase-π
Expressions of miR-124a and miR-30d in the diabetic group and non-diabetic group
The expressions of miR-124a and miR-30d in the diabetic group and non-diabetic group were detected by qRT-PCR. The results showed that the miR-124a expression in the diabetic group was 0.42 fold of that in the non-diabetic group, as the relative expression of miR-124a in the non-diabetic group was defined as 1 (P < 0.05). However, the miR-30d expression in the diabetic group was 1.64 fold of that in the non-diabetic group, as the relative expression of miR-30d in the non-diabetic group was defined as 1 (P < 0.05) (Fig. 1). These results suggested that miR-124a may act as an anti-oncogene while the miR-30d may act as an oncogene in the development of BC with T2DM.
Fig. 1.
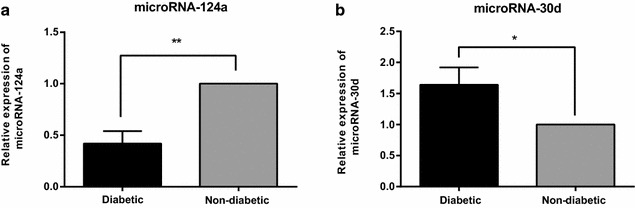
Expressions of miR-124a and miR-30d in the diabetic group and non-diabetic group. Note a miR-124a expression in the diabetic and non-diabetic groups; b miR-30d expression in the diabetic and non-diabetic groups; *P < 0.05; **P < 0.05; miR-124, microRNA-124a; miR-30d, microRNA-30d
Correlations of miR-124a and miR-30d with clinicopathological features in BC patients with T2DM
The Pearson correlation analysis was conducted to explore the correlations between miRs124a/30d and age, BMI, FPG, HbA1c, 2hPG, TC, HDL-C and LDL-C. The correlation analysis showed that the miR-124a expression was positively associated with HDL-C (P < 0.001), while it was negatively associated with age, HbA1c, LDL-C and E2 (all P < 0.05). No significant differences were observed between the miR-124a expression and BMI, FPG, 2hPG or TC (all P > 0.05). Further, the miR-30d expression was negatively correlated with HDL-C (P < 0.001), while it was positively correlated with age, HbA1c, LDL-C and E2 (all P < 0.05). There were no significant differences between the miR-30d expression and BMI, FPG, 2hPG or TC (all P > 0.05), as shown in Table 4. Linear regression analysis showed that the HbA1c, LDL-C, HDL-C and E2 were independent factors for expressions of miR-124a and miR-30d (Tables 5, 6).
Table 4.
Correlation analysis of miR-124a and miR-30d with various biochemical parameters in BC patients with T2DM
| Index | miR-124a | miR-30d | ||
|---|---|---|---|---|
| r value | P value | r value | P value | |
| Age (years) | −0.353 | 0.002 | 0.333 | 0.004 |
| Ratio of menopause | −0.049 | 0.685 | 0.134 | 0.262 |
| BMI (Kg/m2) | −0.172 | 0.148 | 0.218 | 0.066 |
| HDL-C (mmol/L) | 0.698 | <0.001 | −0.731 | <0.001 |
| LDL-C (mmol/L) | −0.754 | <0.001 | 0.786 | <0.001 |
| TC (mmol/L) | −0.071 | 0.554 | 0.069 | 0.564 |
| FPG (mmol/L) | −0.115 | 0.337 | 0.097 | 0.419 |
| 2hPG (mmol/L) | −0.097 | 0.418 | 0.091 | 0.447 |
| HbA1c (%) | −0.443 | <0.001 | 0.496 | <0.001 |
| FIns (uU/mL) | −0.139 | 0.246 | 0.128 | 0.284 |
| HOMA-IS | −0.085 | 0.478 | 0.096 | 0.421 |
| HOMA-IR | −0.160 | 0.179 | 0.139 | 0.243 |
| E2 (pg/mL) | −0.763 | <0.001 | 0.765 | <0.001 |
BMI body mass index, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, TC total cholesterol, FPG fasting blood glucose, 2hPG 2-hour postprandial blood glucose, HbA1c glycosylated hemoglobin, FIns fasting insulin, HOMA-IR homeostasis model assessment of insulin resistance, HOMA-IS HOMA of β-cell insulin secretion, E 2 estradiol
Table 5.
Linear regression analysis of the factors for miR-124a expression
| Model | Unstandardized coefficients | Standardized coefficients | t | Sig. | |
|---|---|---|---|---|---|
| B | Std. error | Beta | |||
| (Constant) | 0.866 | 0.108 | 8.025 | <0.001 | |
| LDL-C | −0.045 | 0.015 | −0.275 | −2.888 | 0.005 |
| Age | −0.002 | 0.001 | −0.125 | −1.977 | 0.052 |
| HDL-C | 0.098 | 0.030 | 0.263 | 3.306 | 0.002 |
| HbA1c | −0.008 | 0.004 | −0.145 | −2.222 | 0.030 |
| E2 | −0.004 | 0.001 | −0.341 | −3.744 | <0.001 |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; E2, estradiol; B: regression coefficient; Std. error: standard error of regression; Sig: significance, P value
Table 6.
Linear regression analysis of the factors for miR-30d expression
| Model | Unstandardized coefficients | Standardized coefficients | t | Sig. | |
|---|---|---|---|---|---|
| B | Std. error | Beta | |||
| (Constant) | 0.617 | 0.223 | 2.764 | 0.007 | |
| LDL-C | 0.121 | 0.032 | 0.321 | 3.806 | <0.001 |
| Age | 0.004 | 0.003 | 0.098 | 1.755 | 0.084 |
| HDL-C | −0.252 | 0.062 | −0.288 | −4.089 | <0.001 |
| HbA1c | 0.026 | 0.008 | 0.194 | 3.364 | 0.001 |
| E2 | 0.007 | 0.002 | 0.287 | 3.561 | 0.001 |
HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, HbA1c glycosylated hemoglobin, E 2 estradiol, B regression coefficient, Std. error standard error of regression, Sig significance, P value
Discussion
In the present study, we aimed to explore the correlations of miR-124a and miR-30d with BC patients with T2DM. We explore the correlations of miR-124a and miR-30d with the clinicopathological features of BC patients with T2DM. We found that the miR-124a expression was positively associated with HDL-C, while miR-30d expression was negatively correlated with HDL-C. Moreover, miR-124a expression was negatively associated with age, HbA1c, LDL-C and E2, while miR-30d expression was positively correlated with age, HbA1c, LDL-C and E2. These results indicated that the levels of HbA1c, LDL-C, HDL-C and E2 were correlated with the expressions of miR-124a and miR-30d, and may be acted as independent factors for expressions of miR-124a and miR-30d.
It was well-known that T2DM is characterized by insulin resistance and impaired insulin secretion caused by insufficiency of pancreatic beta-cells (Mizokami-Stout et al. 2012; Okuno et al. 2013). Additionally, HbA1c may capture the glucose exposure that may relevant to cancer risk and higher HbA1c level may be correlated with a higher risk of cancer incidence and cancer-related mortality (Joshu et al. 2012; Li et al. 2014). Estrogen has also been reported to be involved in the pathogenesis and disease progression of BC and down-regulated estrogen level could be a potential management for most patients with estrogen responsive tumors (Su et al. 2013). Further, the estrogen may promote cell proliferation and inhibits cell apoptosis by modulating gene transcription in estrogen-dependent tumors, and the high serum E2 levels may be associated with specific gene expression patterns in BC tissues (Chalasani et al. 2014; Kim et al. 2013).
Our study results have revealed that the miR-124a expression in BC patients with T2DM was significantly lower than that in BC patients without T2DM. The tumor-related miRs function as tumor suppressors or oncogenes and regulate various aspects of carcinogenesis, including cell proliferation, cell-cycle control, metastasis, and angiogenesis (Landskroner-Eiger et al. 2013; Profumo and Gandellini 2013). MiR-124a is mainly expressed in brains and pancreas, and the over expression of miR-124a in pancreatic β-cells can improve the insulin secretion, but it can reduce the insulin secretion stimulated by high concentration of glucose (Baroukh et al. 2007). The expression and mechanisms of miR-124 have also been investigated in BC, and Han et al. (2013) have found that miR-124 may play a key role in inhibiting the invasion and metastasis of BC cells, probably by directly targeting CD151 genes. MiR-124a overexpression could down-regulate FoxA2 expression, which could bind with PDX1 and ISL1 in the islet amyloid polypeptide (IAPP) promoter, thus decreasing the IAPP levels and inhibiting the apoptosis of β-cells (Jing et al. 2014). Further, Li et al. (2013) have demonstrated that the expression of miR-124 was down-regulated in BC patients, and the miR-124 might be acted as a tumor suppressor in BC through the regulation of FLOT1 gene. The study performed by Dong et al. (2015) has revealed that decreased expression of miR-124 may be associated with advanced TNM stage, lymph node metastasis and poorer pathological differentiation, implying that down-regulation of miR-124 may be an independent unfavorable prognostic factor for BC patients. Further, high levels of insulin are mitogenic for BC cells, and overexpressed insulin receptors are often found in BC patients (Kaplan et al. 2012). Hyperexpression of miR-124a may be impaired glucose-stimulated insulin secretion, and the silencing of the miR-124a resulted in increased expression of target genes for beta-cell function, indicating that an altered miR-124a expression may lead to beta-cell dysfunction in T2DM (Sebastiani et al. 2015). Meanwhile, insulin release from pancreatic beta-cells acts an important role in blood glucose homeostasis, and the pancreatic development is a complex sequential expression of a gamut of transcription factors. Foxa2 deficiency may result in excessive insulin release in response to amino acids and complete loss of glucose-stimulated insulin secretion (Gao et al. 2010; Lantz et al. 2004). Baroukh et al. (2007) have demonstrated that miR-124a may be implicated in the cell differentiation process of beta-cells, and the miR-124a may play as a regulator of a key transcriptional protein network in beta-cells responsible for modulating intracellular signaling by targeting Foxa2 gene. In this regard, we suspected that the lower expression of miR-124a may be involved in the development and progression of BC patients combined with T2DM.
In this study, we found that the miR-30d expression in BC patients with T2DM was significantly higher than that in BC patients without T2DM. Meanwhile, we found that miR-30d expression was positively associated with the levels of HbA1c, LDL-C and E2, and the levels of HbA1c, LDL-C and E2 may be acted as independent factors for expression of miR-30d. Recent evidence has suggested that miR-30d may be acted as a novel antioncogene (Li et al. 2012; Lu et al. 2009; Zhao et al. 2012). In patients with diabetes or with high level of glucose, miR-30d could regulate Map4k4 expression to increase the levels of insulin transcription factors, thus promoting the insulin secretion and reducing TNF-α-induced transcription and production of insulin genes (Tang et al. 2009). It has been reported that miR-30d may suppress renal carcinoma cell proliferation by regulating cyclin E2 expression at a post-transcriptional level (Yu et al. 2014). Tang et al. (2009) have found that the up-regulated expression of miR-30d by glucose may increase the insulin gene expression, while the inhibition of miR-30d may abolish glucose-stimulated insulin gene transcription, which may be a negative regulator of insulin gene expression.
Conclusion
MiR-124a and miR-30d may be correlated with clinicopathological features of BC patients with T2DM. The miR-124a and miR-30d could serve as novel biomarkers for early diagnosis of BC in patients with T2DM. However, the exact mechanism of miR-124a and miR-30d in the progression of BC combined with T2DM was still unclear. Further study based on the target genes of miR-124a or miR-30d need to be conducted to explore the underlying mechanisms of miR-124a and miR-30d on the development of BC combined with T2DM.
Authors’ contributions
YLH, XEC, JXW, CLD, HTC designed the study. YLH, XEC, JXW collated the data, designed and developed the database, carried out data analyses and produced the initial draft of the manuscript. CLD, HTC contributed to drafting the manuscript. All authors have read and approved the final manuscript.
Acknowledgements
We would like to give our sincere appreciation to the reviewers for their helpful comments on this article.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Yu-Ling Han, Email: tonnehan14@163.com.
Xian-E. Cao, Phone: +86-0539-8012793, Email: caoxiane50@163.com
Ju-Xun Wang, Email: wjx_1547@163.com.
Chun-Ling Dong, Email: dongcl_dcl@163.com.
Hong-Tao Chen, Email: red_cht12@163.com.
References
- American Diabetes A (2013) Diagnosis and classification of diabetes mellitus. Diabetes Care 36(Suppl 1):S67–S74 [DOI] [PMC free article] [PubMed]
- American Diabetes A (2014) Diagnosis and classification of diabetes mellitus. Diabetes Care 37(Suppl 1):S81–S90 [DOI] [PubMed]
- Ban KA, Godellas CV. Epidemiology of breast cancer. Surg Oncol Clin N Am. 2014;23(3):409–422. doi: 10.1016/j.soc.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Baroukh N, Ravier MA, Loder MK, Hill EV, Bounacer A, Scharfmann R, Rutter GA, Van Obberghen E. MicroRNA-124a regulates Foxa2 expression and intracellular signaling in pancreatic beta-cell lines. J Biol Chem. 2007;282(27):19575–19588. doi: 10.1074/jbc.M611841200. [DOI] [PubMed] [Google Scholar]
- Bridge G, Monteiro R, Henderson S, Emuss V, Lagos D, Georgopoulou D, Patient R, Boshoff C. The microRNA-30 family targets DLL4 to modulate endothelial cell behavior during angiogenesis. Blood. 2012;120(25):5063–5072. doi: 10.1182/blood-2012-04-423004. [DOI] [PubMed] [Google Scholar]
- Chalasani P, Stopeck A, Clarke K, Livingston R. A pilot study of estradiol followed by exemestane for reversing endocrine resistance in postmenopausal women with hormone receptor-positive metastatic breast cancer. Oncologist. 2014;19(11):1127–1128. doi: 10.1634/theoncologist.2014-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Lin JW, Wu LC, Lai MS, Chuang LM. Oral insulin secretagogues, insulin, and cancer risk in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(7):E1170–E1175. doi: 10.1210/jc.2012-1162. [DOI] [PubMed] [Google Scholar]
- Chen X, He D, Dong XD, Dong F, Wang J, Wang L, Tang J, Hu DN, Yan D, et al. MicroRNA-124a is epigenetically regulated and acts as a tumor suppressor by controlling multiple targets in uveal melanoma. Investig Ophthalmol Visual Sci. 2013;54(3):2248–2256. doi: 10.1167/iovs.12-10977. [DOI] [PubMed] [Google Scholar]
- Deng G, Kakar S, Kim YS. MicroRNA-124a and microRNA-34b/c are frequently methylated in all histological types of colorectal cancer and polyps, and in the adjacent normal mucosa. Oncol Lett. 2011;2(1):175–180. doi: 10.3892/ol.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Ann Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong LL, Chen LM, Wang WM, Zhang LM. Decreased expression of microRNA-124 is an independent unfavorable prognostic factor for patients with breast cancer. Diagn Pathol. 2015;10:45. doi: 10.1186/s13000-015-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. doi: 10.1007/978-94-007-5590-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, Le Lay J, Qin W, Doliba N, Schug J, Fox AJ, Smirnova O, Matschinsky FM, Kaestner KH. Foxa1 and Foxa2 maintain the metabolic and secretory features of the mature beta-cell. Mol Endocrinol. 2010;24(8):1594–1604. doi: 10.1210/me.2009-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginter E, Simko V. Global prevalence and future of diabetes mellitus. Adv Exp Med Biol. 2012;771:35–41. doi: 10.1007/978-1-4614-5441-0_5. [DOI] [PubMed] [Google Scholar]
- Han ZB, Yang Z, Chi Y, Zhang L, Wang Y, Ji Y, Wang J, Zhao H, Han ZC. MicroRNA-124 suppresses breast cancer cell growth and motility by targeting CD151. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2013;31(6):823–832. doi: 10.1159/000350100. [DOI] [PubMed] [Google Scholar]
- He DE, Bai JW, Liu J, Du CW, Huang WH, Zhang GJ. Clinicopathological characteristics and prognosis of breast cancer patients with type 2 diabetes mellitus. Mol Clin Oncol. 2015;3(3):607–612. doi: 10.3892/mco.2015.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing G, Westwell-Roper C, Chen J, Xu G, Verchere CB, Shalev A. Thioredoxin-interacting protein promotes islet amyloid polypeptide expression through mir-124a and foxa2. J Biol Chem. 2014;289(17):11807–11815. doi: 10.1074/jbc.M113.525022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshu CE, Prizment AE, Dluzniewski PJ, Menke A, Folsom AR, Coresh J, Yeh HC, Brancati FL, Platz EA, et al. Glycated hemoglobin and cancer incidence and mortality in the Atherosclerosis in Communities (ARIC) Study, 1990–2006. Int J Cancer. 2012;131(7):1667–1677. doi: 10.1002/ijc.27394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MA, Pekkolay Z, Kucukoner M, Inal A, Urakci Z, Ertugrul H, Akdogan R, Firat U, Yildiz I, et al. Type 2 diabetes mellitus and prognosis in early stage breast cancer women. Med Oncol. 2012;29(3):1576–1580. doi: 10.1007/s12032-011-0109-4. [DOI] [PubMed] [Google Scholar]
- Kim JY, Han W, Moon HG, Ahn SK, Kim J, Lee JW, Kim MK, Kim T, Noh DY. Prognostic effect of preoperative serum estradiol level in postmenopausal breast cancer. BMC Cancer. 2013;13:503. doi: 10.1186/1471-2407-13-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskroner-Eiger S, Moneke I, Sessa WC (2013) miRNAs as modulators of angiogenesis. Cold Spring Harbor Perspect Med 3(2):a006643 [DOI] [PMC free article] [PubMed]
- Lantz KA, Vatamaniuk MZ, Brestelli JE, Friedman JR, Matschinsky FM, Kaestner KH. Foxa2 regulates multiple pathways of insulin secretion. J Clin Investig. 2004;114(4):512–520. doi: 10.1172/JCI21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Kaur S, Greshock J, Lassus H, Zhong X, Wang Y, Leminen A, Shao Z, Hu X, et al. A combined array-based comparative genomic hybridization and functional library screening approach identifies mir-30d as an oncomir in cancer. Cancer Res. 2012;72(1):154–164. doi: 10.1158/0008-5472.CAN-11-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Luo J, Wang B, Wang D, Xie X, Yuan L, Guo J, Xi S, Gao J, et al. Microrna-124 targets flotillin-1 to regulate proliferation and migration in breast cancer. Molecular cancer. 2013;12:163. doi: 10.1186/1476-4598-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XL, Li JJ, Guo YL, Zhu CG, Xu RX, Li S, Qing P, Wu NQ, Jiang LX, et al. Relationship of glycated hemoglobin levels with myocardial injury following elective percutaneous coronary intervention in patients with type 2 diabetes mellitus. PLoS ONE. 2014;9(7):e101719. doi: 10.1371/journal.pone.0101719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Li J, Wei W, Wang L, Zhang Y, Li J, Wang C, Sun S. Association between diabetes mellitus and breast cancer risk: a meta-analysis of the literature. Asian Pac J Cancer Prevent. 2011;12(4):1061–1065. [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu Y, Ryan SL, Elliott DJ, Bignell GR, Futreal PA, Ellison DW, Bailey S, Clifford SC (2009) Amplification and overexpression of Hsa-miR-30b, Hsa-miR-30d and KHDRBS3 at 8q24.22-q24.23 in medulloblastoma. PLoS ONE 4(7):e6159 [DOI] [PMC free article] [PubMed]
- Luo J, Virnig B, Hendryx M, Wen S, Chelebowski R, Chen C, Rohan T, Tinker L, Wactawski-Wende J, et al. Diabetes, diabetes treatment and breast cancer prognosis. Breast Cancer Res Treatment. 2014;148(1):153–162. doi: 10.1007/s10549-014-3146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizokami-Stout K, Cree-Green M, Nadeau KJ. Insulin resistance in type 2 diabetic youth. Curr Opin Endocrinol Diabetes Obesity. 2012;19(4):255–262. doi: 10.1097/MED.0b013e3283557cd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno A, Kaji N, Takahashi A, Nagakubo D, Ohno-Ichiki K, Shirai M, Asai F. Role of insulin resistance in the pathogenesis and development of type 2 diabetes in WBN/Kob-Lepr(fa) rats. J Vet Med Sci Jpn Soc Vet Sci. 2013;75(12):1557–1561. doi: 10.1292/jvms.13-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppong BA, Pharmer LA, Oskar S, Eaton A, Stempel M, Patil S, King TA. The effect of metformin on breast cancer outcomes in patients with type 2 diabetes. Cancer Med. 2014;3(4):1025–1034. doi: 10.1002/cam4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Ren H, Ma Y, Liu D, Yu B, Ji L, Pan L, Li J, Yang L, et al. High-density lipoprotein of patients with type 2 diabetes mellitus elevates the capability of promoting migration and invasion of breast cancer cells. Int J Cancer. 2012;131(1):70–82. doi: 10.1002/ijc.26341. [DOI] [PubMed] [Google Scholar]
- Pn M. World Medical Association publishes the Revised Declaration of Helsinki. Nat Med J India. 2014;27(1):56. [PubMed] [Google Scholar]
- Profumo V, Gandellini P. MicroRNAs: cobblestones on the road to cancer metastasis. Crit Rev Oncog. 2013;18(4):341–355. doi: 10.1615/CritRevOncog.2013007182. [DOI] [PubMed] [Google Scholar]
- Sebastiani G, Po A, Miele E, Ventriglia G, Ceccarelli E, Bugliani M, Marselli L, Marchetti P, Gulino A, et al. MicroRNA-124a is hyperexpressed in type 2 diabetic human pancreatic islets and negatively regulates insulin secretion. Acta Diabetol. 2015;52(3):523–530. doi: 10.1007/s00592-014-0675-y. [DOI] [PubMed] [Google Scholar]
- Shikata K, Ninomiya T, Kiyohara Y. Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer Sci. 2013;104(1):9–14. doi: 10.1111/cas.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB et al (2003) Staging system for breast cancer: revisions for the 6th edition of the AJCC cancer staging manual. Surg Clin N Am 83(4):803–819 [DOI] [PubMed]
- Su HI, Sue LY, Flatt SW, Natarajan L, Patterson RE, Pierce JP. Endogenous estradiol is not associated with poor physical health in postmenopausal breast cancer survivors. J Women Health. 2013;22(12):1043–1048. doi: 10.1089/jwh.2013.4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Muniappan L, Tang G, Ozcan S. Identification of glucose-regulated miRNAs from pancreatic beta cells reveals a role for miR-30d in insulin transcription. RNA. 2009;15(2):287–293. doi: 10.1261/rna.1211209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, Li G, Wang L. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130(7):1639–1648. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhong X, Tanyi JL, Shen J, Xu C, Gao P, Zheng TM, DeMichele A, Zhang L. mir-30d Regulates multiple genes in the autophagy pathway and impairs autophagy process in human cancer cells. Biochem Biophys Res Commun. 2013;431(3):617–622. doi: 10.1016/j.bbrc.2012.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Lin X, Wang F, Zhang B, Wang W, Shi H, Zou B, Zhao J. Proliferation inhibition and the underlying molecular mechanisms of microRNA-30d in renal carcinoma cells. Oncol Lett. 2014;7(3):799–804. doi: 10.3892/ol.2013.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Mohan R, Ozcan S, Tang X. MicroRNA-30d induces insulin transcription factor MafA and insulin production by targeting mitogen-activated protein 4 kinase 4 (MAP4K4) in pancreatic beta-cells. J Biol Chem. 2012;287(37):31155–31164. doi: 10.1074/jbc.M112.362632. [DOI] [PMC free article] [PubMed] [Google Scholar]


