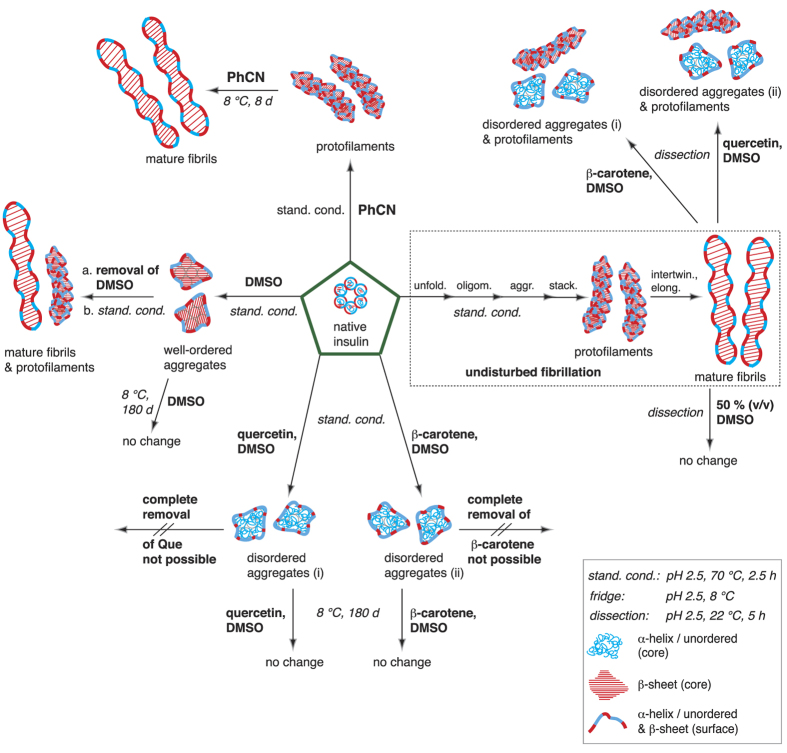
Figure 1. Overview of all compounds characterized in the presented insulin fibrillation inhibition and fibril dissection experiments.
The structures were assigned after characterization with AFM, TERS and standard Raman spectroscopy. TERS and conventional Raman spectroscopy were used to identify the secondary structures of the surface and core. Legend: α-helix/unordered structures (blue tangles), β-sheet structures (red bands), and mixtures of both conformations (red-blue bands). All experiments started from native insulin (green pentagons). Under undisturbed standardized fibrillation conditions (pH 2.5, 70 °C, 2.5 h, dotted rectangle), mature fibrils were obtained; in the presence of PhCN under standard conditions, protofilaments were identified, which transformed into mature fibrils (pH 2.5, 8 °C) within 8 days (d); in the presence of DMSO under standard conditions, aggregates were obtained with a β-sheet core, whereas on the surface, α -helix/unordered structures and β-sheet structures were identified; after the complete removal of DMSO, the fibrillation of the aggregates under standard conditions resulted in protofilaments and mature fibrils; in the presence of β-carotene or Que in DMSO, insulin did not fibrillize under standard conditions, but disordered aggregates (i) and (ii) were formed, which were stable in the solution at 8 °C for months; mature fibrils could be dissected (dissection: pH 2.5, 22 °C, 5 h) with β-carotene or Que in DMSO, yielding disordered aggregates (i) and (ii); mature fibrils could not be dissected when DMSO was added to the pH 2.5 gel at a 50% (v/v) ratio.