Abstract
Study objective
Induction doses of etomidate during rapid sequence intubation (RSI) cause transient adrenal dysfunction, but its clinical significance on trauma patients is uncertain. Ketamine has emerged as an alternative for RSI induction. Among adult trauma patients emergently intubated, we compared clinical outcomes among those induced with etomidate and ketamine.
Methods
The study entailed a retrospective evaluation of a four-year (January 2011–December 2014) period spanning an institutional protocol switch from etomidate to ketamine as the standard induction agent for adult trauma patients undergoing RSI in the ED of an academic Level I trauma center. The primary outcome was hospital mortality evaluated with multivariable logistic regression adjusted for age, vital signs, and injury severity and mechanism. Secondary outcomes included intensive care unit (ICU)-free days and ventilator-free days (VFD) evaluated with multivariable ordered logistic regression using the same covariates.
Results
The analysis included 968 patients, including 526 with etomidate and 442 with ketamine. Hospital mortality was 20.4% among patients induced with ketamine compared to 17.3% among those induced with etomidate (aOR: 1.41; 95% CI: 0.92, 2.16). Patients induced with ketamine had similar ICU-free-days (aOR: 0.80; 95% CI: 0.63, 1.00) and VFDs (aOR: 0.96; 95% CI: 0.76, 1.20) as compared to patients induced with etomidate.
Conclusion
In this analysis spanning an institutional protocol switch from etomidate to ketamine as the standard RSI induction agent for adult trauma patients, patient-centered outcomes were similar for patients who received etomidate and ketamine.
Keywords: intubation, trauma, etomidate, ketamine
INTRODUCTION
Background
Trauma is the leading cause of death for Americans under 45 years old, and accounts for 30 million emergency department (ED) visits and three million hospitalizations annually in the United States (US).1,2 Clinical practice guidelines recommend rapid sequence intubation (RSI) as the procedure of choice for intubating acutely-injured patients.3 Due to their rapid onset and favorable hemodynamic effects, both etomidate and ketamine are used for RSI induction in trauma.4–10 However, whether one agent should be preferred over the other for RSI of trauma patients remains unclear.
Importance
Induction doses of etomidate inhibit the 11-β hydroxylase enzyme and cause transient adrenal suppression that may negatively impact severely injured patients.11–16 Although ketamine has emerged as an alternative to etomidate for RSI in trauma, experience with ketamine in this setting is limited due to historical concerns about it increasing intracranial pressure, and evidence suggesting it has a direct myocardial depressant effect, which may lead to complications in critically ill patients with diminished physiologic reserve.17–21
Goals of This Investigation
Based on data suggesting etomidate-induced adrenal dysfunction may be associated with adverse outcomes in trauma,22–25 in December 2012 our institution changed the standard induction agent for ED RSI of trauma patients from etomidate to ketamine. Using this systematic protocol change, we compared the morbidity and mortality of trauma patients intubated with etomidate and ketamine during a four-year period spanning this practice change.
MATERIALS AND METHODS
Study Design and Setting
This was a retrospective analysis of data collected at an academic, tertiary care, Level I trauma center in the US with approximately 70,000 adult ED visits and 3,600 acute trauma admissions annually. The study period was January 1, 2011 through December 31, 2014. Adult trauma patients were intubated by emergency physicians using a standardized clinical protocol. Prior to December 2012, etomidate was the on-protocol, standard induction agent for ED RSI of trauma patients. In December 2012, ketamine replaced etomidate as the on-protocol induction agent. There were no other changes to the RSI protocol during this time. Recommended induction doses were: etomidate 0.3 mg/kg; and ketamine 1–2 mg/kg. Succinylcholine was the standard on-protocol RSI paralytic throughout the study period. All treatment decisions were made by treating clinicians independent of this study; throughout the study period, treating clinicians had the ability to select an off-protocol induction agent based on clinical discretion. The study was approved by the local institutional review board (IRB #150666) with waiver of informed consent.
Selection of Participants
Patients ≥18 years old were included if they presented with acute trauma and were intubated in the ED using either etomidate or ketamine for RSI induction. Several a priori subgroups were also identified, including patients with: traumatic brain injury (TBI); Glasgow Coma Scale (GCS) < 15 at presentation; penetrating trauma; “major trauma,” defined as an Injury Severity Score (ISS) > 15; and systolic blood pressure (SBP) < 100 mm Hg at presentation. These subgroups were selected based on the hypothesis that patients with TBI and severe trauma may be particularly vulnerable to etomidate-induced adrenal suppression.26,27 TBI was defined as intracranial hemorrhage, diffuse axonal injury, or shear injury identified by an attending radiologist on the first head computed tomography scan after presentation.
Exposure Groups
As detailed in the Analysis section below, two separate analyses were conducted and the exposure definitions varied per analyses. In the primary analysis, patients who received ketamine were compared to those who received etomidate, regardless of which agent was the standard RSI on-protocol induction agent at the time of intubation. A secondary, quasi-experimental analysis assessed the impact of the RSI protocol change from etomidate to ketamine; for this analysis, outcomes from patients intubated after the induction agent protocol switch in December 2012 (the ketamine period) were compared to those intubated before the protocol switch (the etomidate period), regardless of the induction agent received.
Outcomes
The primary outcome was hospital mortality, defined as death in the ED or during the index hospitalization following RSI in the ED. Secondary outcomes included: days alive and outside an ICU between ED RSI and 28 days later (ICU-free days); days alive and free of invasive mechanical ventilation between the time of ED RSI and 28 days later (ventilator-free days); days alive and free of vasopressor support between ED RSI and 28 days later (vasopressor-free days); units of packed red blood cells (PRBCs) transfused in the first 48 hours; hospital-acquired sepsis to day 28, defined as ≥2 systemic inflammatory response syndrome criteria with confirmed/suspected source of infection; time to hospital discharge; and hazard of hospital death. Consistent with prior literature on the “-free day” composite outcomes,28 patients who died before day 28 were considered to have experienced zero ICU-free days, ventilator-free days, and vasopressor-free days, and those discharged or transferred prior to day 28 were assumed to have no additional days in the ICU, on the ventilator, or on vasopressors after discharge or transfer.
Additional outcomes used to assess intubating conditions during RSI included: first-pass intubation success; need for rescue surgical airway; and peri-intubation cardiac arrest, defined as cardiac arrest within one hour of induction agent administration.
Data Collection
Data were abstracted from the electronic medical record, our medical center enterprise data warehouse, and the institution’s Trauma Registry of the American College of Surgeons (TRACS) database. Data were collected using high-quality chart review standards29 and managed using the REDCap electronic data capture tool.30 After case identification and extraction from our data registries, medical record data were collected by a single investigator (CPU), who was a senior medical student trained on the study protocol and variable definitions by the senior investigator. A second investigator (WHS) independently reviewed a random 10% subset of included patients, and inter-rater agreement between the two reviewers was calculated for the primary exposure variable (induction agent) and the primary outcome (hospital mortality). Chart abstractors were not blinded to the study’s purpose.
Analysis
Primary Analysis
For the primary analysis, exposure groups were defined by the induction agent administered to each patient. Multivariable logistic regression models were used to evaluate the association between induction agent (ketamine vs etomidate as a referent) and hospital mortality. Model covariates, selected a priori based on literature review31,32 and mechanistic plausibility for confounding, included: age; gender; ED presentation heart rate, SBP, and GCS; ISS; and injury mechanism (blunt vs penetrating). Age, heart rate, and SBP had non-linear relationships with mortality and were modeled with four-knot restricted cubic spline functions.33 The association between induction agent and hospital mortality was also estimated for each subgroup using this model. For these subgroup analyses, interaction terms between the subgroup and induction agent were examined, and estimates of association between induction agent and hospital mortality were calculated using linear combinations of coefficients from the regression models that included the interaction terms.34
Multivariable models for secondary outcomes were constructed with the same covariates used in the mortality model. ICU-free days, ventilator-free days, vasopressor-free days, and units of PRBCs transfused were modeled with ordered logistic regression. Hospital-acquired sepsis was modeled with logistic regression. Time to hospital discharge was modeled with proportional hazard regression while considering hospital death as a competing risk event. Hazard of hospital death was modeled with proportional hazard regression with hospital discharge treated as a censoring event.
Secondary Analysis
Association between the implementation of ketamine as the new on-protocol induction agent for ED RSI of trauma patients and hospital mortality was evaluated using an interrupted time series analysis.35,36 The four-year study period was divided into 24 two-month intervals (bimonths). The etomidate period (before the institutional protocol switch) was defined as January 2011 to October 2012 (11 bimonths). November 2012 to February 2013 (2 bimonths) was defined as a transition period as the protocol change was introduced and implemented, and was excluded from analysis. The ketamine period (after the institutional protocol switch) was defined as March 2013 to December 2014 (11 bimonths). A segmented linear regression model was constructed to analyze changes in hospital mortality over time.37 The dependent variable for the model was the proportion of patients who died (hospital mortality) during each bimonthly interval. Independent variables included a term for the time of protocol change from etomidate to ketamine, and terms for secular trends in the etomidate and ketamine periods. Model output provided estimates for the change in hospital mortality over time during the etomidate and ketamine periods, as well as the immediate change in hospital mortality associated with the protocol switch.
Statistical analyses were conducted with Stata (Version 12.1; StataCorp LP, College Station, TX) and R (Version 3.2.3). We evaluated the overall calibration of our multivariable logistic regression model using the Hosmer-Lemeshow goodness-of-fit test. Linear regression model assumptions were assessed through standard examination of residuals and evaluation of the potential autocorrelation of error terms in our time-series data. These assessments indicated that our logistic regression model fit the data well, and no major departures from linear regression model assumptions were noted.
RESULTS
Characteristics of Study Subjects
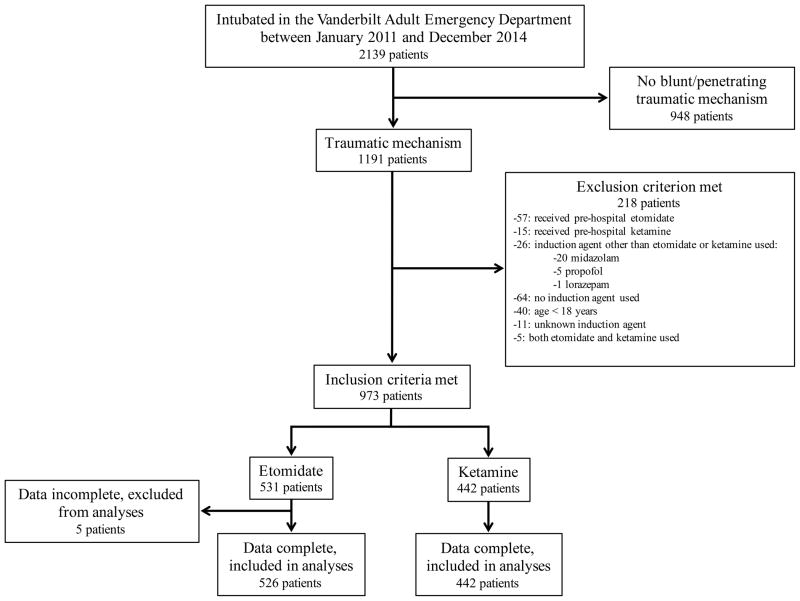
The study population included 968 patients, including 526 (54%) who received etomidate and 442 (46%) who received ketamine (Figure 1). The median dose was 20 mg (IQR: 15–20) for etomidate and 150 mg (IQR: 150–150) for ketamine.
Figure 1.
Flow diagram for generation of the study population.
Characteristics were similar for patients who received etomidate and ketamine, including age, vital signs, ISS, injury mechanism, and Elixhauser comorbidity summary score (Table 1). Administration of at least one dose of systemic steroids during the 28 days following RSI was similar between the etomidate (15.2%) and ketamine (16.1%) groups (0.9% absolute difference; 95% CI: −3.7%, 5.5%). Inter-rater agreement between the two investigators performing data collection was 100% (κ= 1.00; SE: 0.10) for both induction agent and hospital mortality.
Table 1.
Patient characteristics by induction agent.
| Characteristic | Etomidate (n=526) | Ketamine (n=442) |
|---|---|---|
| Demographics | ||
| Age (years), median (IQR) | 39.8 (26–57) | 37.1 (26.6–53.5) |
| Gender (female), n (%) | 139 (26.4) | 122 (27.6) |
| Race (white), n (%) | 392 (74.5) | 324 (73.3) |
| Race (black), n (%) | 103 (19.6) | 101 (22.9) |
| Race (other), n (%) | 31 (5.9) | 17 (3.8) |
| Patient Characteristics at ED Presentation | ||
| Glasgow Coma Scale, median (IQR) | 13 (7–15) | 12 (5–15) |
| Systolic blood pressure (mm Hg), median (IQR) | 130 (107–150) | 122 (100–143) |
| Diastolic blood pressure* (mm Hg), median (IQR) | 80 (64–90) | 80 (62–90) |
| Heart rate (beats/min), median (IQR) | 103 (83–119) | 103 (88–120) |
| Respiratory rate* (breaths/min), median (IQR) | 20 (16–24) | 19 (16–24) |
| Injury Mechanisms | ||
| Any Blunt Mechanism, n (%) | 441 (83.8) | 361 (81.7) |
| Motor vehicle crash, n (%) | 217 (41.3) | 170 (38.5) |
| Fall, n (%) | 88 (16.7) | 58 (13.1) |
| Motorcycle crash, n (%) | 52 (9.9) | 49 (11.1) |
| Pedestrian vs motor vehicle, n (%) | 32 (6.1) | 32 (7.2) |
| Assault, n (%) | 33 (6.3) | 27 (6.1) |
| Crush, n (%) | 11 (2.1) | 9 (2) |
| Bicycle crash, n (%) | 4 (0.8) | 4 (0.9) |
| Other, n (%) | 4 (0.8) | 12 (2.7) |
| Any Penetrating Mechanism, n (%) | 85 (16.2) | 81 (18.3) |
| Gunshot wound, n (%) | 61 (11.6) | 59 (13.4) |
| Stab, n (%) | 20 (3.8) | 19 (4.3) |
| Impalement, n (%) | 3 (0.6) | 0 (0) |
| Other, n (%) | 1 (0.2) | 3 (0.7) |
| Specific Injuries | ||
| Acute adrenal injurya, n (%) | 29 (5.5) | 7 (1.6) |
| Traumatic brain injuryb, n (%) | 178 (33.8) | 134 (30.3) |
| Injury Severity | ||
| Injury Severity Scorec, median (IQR) | 22 (13–33) | 22 (13–29) |
| APACHE II score d*, median (IQR) | 22 (17–27) | 21 (16–26) |
| Presentation SBP < 100 mm Hg, n (%) | 117 (22.2) | 121 (27.4) |
| Elixhausere summary score*, median (IQR) | 5 (0.5–11) | 5 (1–9) |
Abbreviations: IQR, interquartile range; CPR, cardiopulmonary resuscitation; ED, emergency department; APACHE, Acute Physiology and Chronic Health Evaluation; SBP, systolic blood pressure
Defined as focal adrenal hematoma, gross or focal adrenal hemorrhage, and/or active adrenal extravasation per attending radiologist read of initial computed tomography.
Defined as intracranial bleed (epidural, subdural, subarachnoid, intra-axial [intraparenchymal and/or intraventricular]) and/or shear/diffuse axonal injury per attending radiologist read of initial head computed tomography.
Injury Severity Score: score range is 0 to 75. Increasing score indicates greater injury severity and correlates with risk of death. A score greater than 15 is typically defined as major trauma.
APACHE II: score range is 0 to 71. A higher score correlates with higher risk of death.
Elixhauser summary score: comorbidity score, based on the presence of 30 comorbidities, that ranges from −19 to 89; higher score correlates with increased risk of hospital mortality.
Data not complete for all 968 patients. Patients with missing data: 109 (11.3%) for presentation diastolic blood pressure; 121 (12.5%) for presentation respiratory rate; 49 (5.1%) for APACHE II; 275 (28.4%) for Elixhauser summary score.
Main Results
Primary Analysis
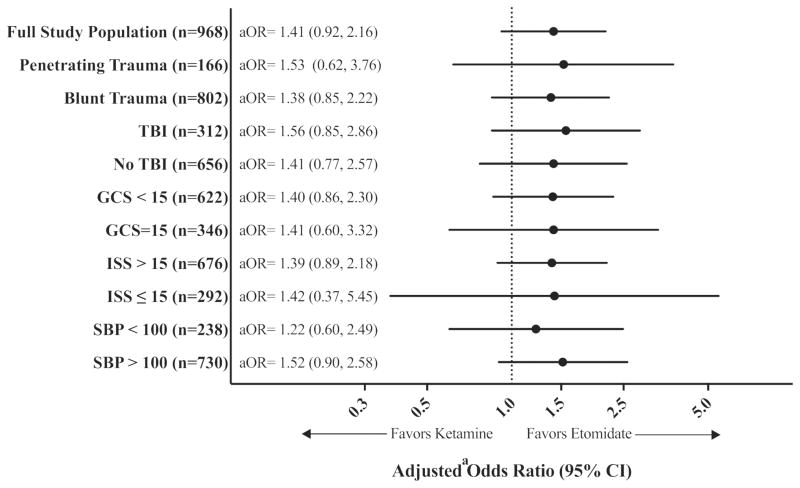
Overall, 181 (18.7%) patients experienced hospital mortality, including 90 (20.4%) induced with ketamine compared with 91 (17.3%) induced with etomidate (aOR: 1.41; 95% CI: 0.92, 2.16; Table 2). This finding was consistent across all subgroups (Figure 2).
Table 2.
Clinical outcomes by induction agent received (ketamine vs etomidate).
| Outcome | Etomidate (n=526) | Ketamine (n=442) | Regression Model (output) | Unadjusted Result (95% CI) [etomidate referent] | Adjusted Resulta (95% CI) [etomidate referent] |
|---|---|---|---|---|---|
| Primary | |||||
| Hospital mortality, n (%) | 91 (17.3) | 90 (20.4) | Logistic (odds ratio) | 1.22 (0.88, 1.69) | 1.41 (0.92, 2.16) |
| Secondary | |||||
| ICU-free days to day 28, median (IQR) | 24.5 (13.3–27.2) | 24.8 (11.2–27.0) | Ordered Logistic (odds ratio) | 0.93 (0.75, 1.16) | 0.80 (0.63, 1.00) |
| Ventilator-free days to day 28, median (IQR) | 26.4 (16.0–27.4) | 26.6 (14.3–27.5) | Ordered Logistic (odds ratio) | 1.07 (0.86, 1.33) | 0.96 (0.76, 1.20) |
| Vasopressor-free days to day 28, median (IQR) | 27 (26–28) | 27 (25–28) | Ordered Logistic (odds ratio) | 0.86 (0.68, 1.08) | 0.74 (0.58, 0.95) |
| PRBC units transfused to 48 hours, median (IQR) | 0 (0–4) | 0 (0–5) | Ordered Logistic (odds ratio) | 1.19 (0.94, 1.51) | 1.14 (0.87, 1.49) |
| Hospital-acquired sepsis, n (%) | 146 (27.8) | 99 (22.4) | Logistic (odds ratio) | 0.75 (0.56, 1.01) | 0.72 (0.52, 0.99) |
| Time to hospital discharge (days), median (IQR) | 7.5 (2.8–15.7) | 6.7 (2.5–13.9) | Proportional Hazard (hazard ratio) | 1.17 (1.01, 1.35) | 1.10 (0.95, 1.27) |
| Hazard of hospital death | n/a | n/a | Proportional Hazard (hazard ratio) | 1.26 (0.94, 1.69) | 1.15 (0.84, 1.56) |
Abbreviations: CI, confidence interval; ICU, Intensive Care Unit; IQR, interquartile range; PRBC, packed red blood cell
All models adjusted for: age; gender; emergency department presentation heart rate, systolic blood pressure, and Glasgow Coma Scale score; Injury Severity Score; and injury mechanism.
Figure 2.
Association between induction agent received (ketamine vs etomidate) and hospital mortality by subgroup population.
Figure Abbreviations: TBI, traumatic brain injury; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; SBP, systolic blood pressure (mm Hg); aOR, adjusted odds ratio; CI, confidence interval. a Adjusted for: age; gender; emergency department presentation heart rate, systolic blood pressure, and Glasgow Coma Scale score; Injury Severity Score; and injury mechanism
Compared with etomidate, ketamine was associated with lower odds of hospital-acquired sepsis (aOR: 0.72; 95% CI: 0.52, 0.99), but fewer vasopressor-free days (aOR: 0.74; 95% CI: 0.58, 0.95), which corresponds to longer duration of vasopressor use. Otherwise, secondary outcomes were similar (Table 2; Web Appendix Figure E1).
Peri-intubation outcomes were also similar in patients who received ketamine compared to those who received etomidate, including first-pass intubation success (93.7% vs 93.9%, absolute risk difference: −0.2%; 95% CI: −3.3%, 2.8%), need for rescue surgical airway (0.2% vs 0.2%, absolute risk difference: 0; 95% CI: −0.5%, 0.6%), and peri-intubation cardiac arrest (2.7% vs 1.5%, absolute risk difference: 1.2%; 95% CI: −0.8%, 3.0%).
Secondary Analysis
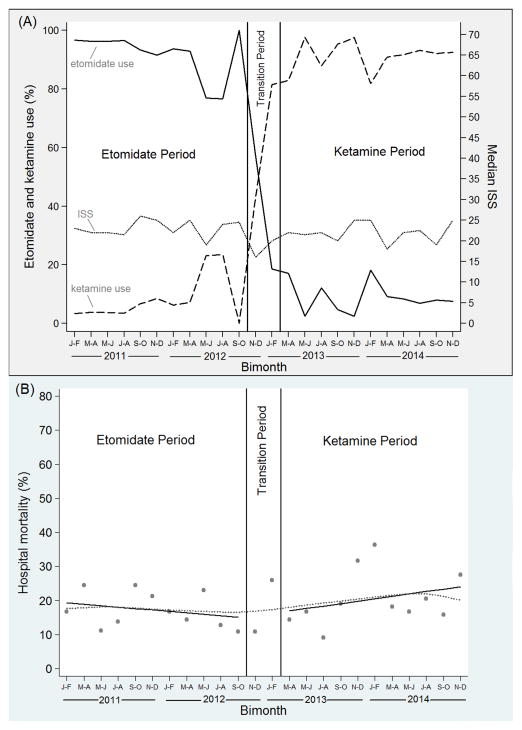
During the “etomidate period” prior to the institutional protocol switch from etomidate to ketamine, 508 patients were included in the study, including 466 (91.7%) who received etomidate and 42 (8.3%) who received ketamine (Figure 3A). During the transition period, there were 64 total patients, including 37 (57.8%) who received etomidate and 27 (42.2%) who received ketamine. During the “ketamine period” following the protocol switch, 396 patients were included in the study, including 362 (91.4%) who received ketamine and 34 (8.6%) who received etomidate (Figure 3A). ISS for included patients remained similar across the entire study period; the median ISS was 22 (IQR: 13–34) during the etomidate period, and 22 (IQR: 13–29) during the ketamine period (Figure 3A). Patient characteristics were similar in the etomidate and ketamine periods (Web Appendix Table E1).
Figure 3.
Interrupted time series analysis. (A) Percentage of patients receiving etomidate and ketamine [left axis] and median Injury Severity Score (ISS) [right axis] during bimonthly intervals. (B) Segmented regression analysis displaying hospital mortality during bimonthly intervals. Each dot represents the percentage of patients intubated during specific bimonthly intervals who died. The dotted line represents a lowess curve fit to the data. Solid dark lines represent best-fit linear regression lines during the etomidate period (slope: −0.4, 95% CI: −1.8, 1.0) and ketamine period (slope: 0.7, 95% CI: −0.7, 2.1).
Overall, unadjusted hospital mortality was 17.3% during the etomidate period and 20.7% during the ketamine period (absolute risk difference: 3.4%; 95% CI: −1.8%, 8.6%). In segmented regression analysis, there was no significant trend in hospital mortality during the etomidate period (−0.4% absolute change per bimonth; 95% CI: −1.8%, 1.0%), and no significant change in hospital mortality immediately associated with the protocol switch from etomidate to ketamine (1.2% absolute change; 95% CI: −11.3%, 13.6%). Furthermore, hospital mortality trends in the ketamine and etomidate periods did not significantly differ from one another (1.1% absolute change; 95% CI: −0.8%, 3.1%) (Figure 3B). The segmented linear regression was repeated with adjustment for median ISS, and results were unchanged compared to the unadjusted analysis.
LIMITATIONS
Our study has some limitations. First, although several techniques were used to account for potential confounders, residual confounding is still possible. Second, while our study is larger than all previously published studies comparing etomidate and ketamine for RSI in trauma, our sample size did not allow for detection of small, but potentially important, differences in mortality. Duration of the study period was selected based on the timing of the RSI protocol switch from etomidate to ketamine at the study institution. We included patients intubated during the two-year period before the protocol switch and the two-year period after the switch. This study duration was selected to balance considerations about sample size and practice variation over time that could confound the relationship between induction agent and hospital mortality. A longer study period would have resulted in a larger sample size but also potentially greater susceptibility to temporal variation in routine clinical practice administered to etomidate patients (predominantly treated in the early part of the study period) and ketamine patients (predominantly treated in the later part of the study period). Third, due to the nature of the protocol switch, our main analysis was based on non-concurrent cohorts and is potentially susceptible to temporal changes in practice or patients. However, our groups were similar in measured characteristics and differences were accounted for analytically. Furthermore, our secondary analysis with an interrupted time series approach statistically adjusted for temporal trends in mortality. Fourth, we did not directly measure adrenal function in this study. However, it has been well established that induction doses of etomidate transiently impair cortisol synthesis;38–40 in the current study, we focused on evaluating whether ketamine was associated with an improvement in clinical outcomes compared to etomidate, with its known effect on adrenal function. Fifth, full induction doses of etomidate (~0.3 mg/kg) and ketamine (1–2 mg/kg) were administered to nearly all patients in this study, and we are unable to assess if lower doses may have led to better outcomes in hypotensive patients. Lastly, our study was conducted at a single academic Level I trauma center, and further evaluation in other settings is indicated.
DISCUSSION
In this study of 968 adult trauma patients intubated at one institution during a four-year period spanning an institutional protocol switch from etomidate to ketamine for ED RSI induction, use of ketamine compared with etomidate was not associated with an improvement in clinical outcomes, including hospital mortality, ICU-free days, ventilator-free days, vasopressor-free days, and transfusion requirements. Subgroup analyses of the most severely injured patients also consistently failed to show a mortality benefit for ketamine compared to etomidate.
The potential association between induction agent and hospital mortality was evaluated using two analyses: traditional multivariable regression techniques adjusting for potential patient-level confounders, and a quasi-experimental interrupted times series analysis of the institutional protocol switch. These distinct but complementary analytical strategies led to similar conclusions—the use of ketamine was not associated with an improvement in hospital mortality compared to etomidate.
The National Emergency Airway Registry, a large multicenter surveillance group, demonstrated that etomidate was used in more than 90% of RSIs in the ED during the last decade, while ketamine was used for only 1%.41 However, the safety of etomidate has been repeatedly questioned due to transient adrenal dysfunction it causes following induction. Several small studies have suggested etomidate may be associated with poor outcomes in trauma patients.22–25,42 At the same time, ketamine emerged as an attractive RSI induction agent due to new data showing it does not increase intracranial pressure in brain-injured patients,20,21 and increased experience with its use for procedural sedation. Prior published data on the use of ketamine for RSI induction of trauma patients are limited. The KETASED trial,43 in which critically ill adults undergoing RSI were randomized to etomidate or ketamine, included a subgroup of 104 trauma patients; there was a non-significant trend toward higher mortality in patients randomized to ketamine in this subgroup with trauma. Results of our study and the KETASED trial are similar and do not support concerns that etomidate-induced adrenal dysfunction is associated with worse patient-centered outcomes in trauma patients compared to ketamine.
Interestingly, ketamine was associated with lower odds of hospital-acquired sepsis compared to etomidate in our study. Although this lower prevalence of infection did not translate into differences in mortality or duration of ICU or ventilator use, susceptibility to infection is a potential concern related to etomidate-associated adrenal dysfunction that will be important to evaluate in future trials.25 Hospital-acquired sepsis was one of multiple secondary outcomes in our study, and this finding requires independent confirmation.
Ketamine was associated with fewer vasopressor-free days, that is, a longer duration of vasopressor use. Ketamine has indirect sympathomimetic effects by inhibiting reuptake of endogenous catecholamines, but also has direct myocardial depressant effects17,44 that may decrease ventricular contractility in critically-ill patients.19,45 It has been proposed that the negative inotropic effects of ketamine may outweigh the sympathomimetic effects in patients with depleted physiologic reserve, and potentially lead to increased mortality and longer duration of organ dysfunction in these patients.18,46 This finding also requires independent confirmation in future studies.
Due to the high volume of RSI procedures performed among trauma patients, even a small difference in mortality risk between induction agents could lead to substantial differences in survival on a population level. There is equipoise about the best RSI induction agent for trauma patients, and thus, randomized controlled studies powered to detect relatively small but clinically important mortality differences are indicated. In the meantime, results from this quasi-experimental study can help inform clinical practice. Clinicians using etomidate for RSI of trauma patients should be reassured that ketamine was not associated with an improvement in mortality or other patient-centered outcomes in our study.
In conclusion, this study of 968 adult trauma patients who underwent ED RSI does not support the hypothesis that induction with ketamine is associated with more favorable patient-centered outcomes than etomidate.
Supplementary Material
Figure E1. Survival curves displaying the percentage of patients alive over time by induction agent received. Observation time was censored at hospital discharge. The adjusted hazard ratio (aHR) compares the hazard of death for patients who received ketamine to those who received etomidate (referent). Model covariates included: age, gender, heart rate, systolic blood pressure, Glasgow Coma Scale score, Injury Severity Score, and injury mechanism.
Table E1. Patient characteristics by period in relation to the protocol switch from etomidate to ketamine for ED RSI of trauma patients. The etomidate period included the 11 bimonths between January 2011 and October 2012. The two bimonths between November 2012 and February 2013 were considered a transition period. The ketamine period included the 11 bimonths between March 2013 and December 2014.
Acknowledgments
We thank Melissa Smith, MSN, RN and Paula Bergon, CPC for assistance in accessing the institutional TRACS database.
Sources of Funding: This work was funded by the Vanderbilt University School of Medicine Medical Scholars Program and the Vanderbilt University CTSA grant (TL1TR000447). Dr. Grijalva was supported in part by R01AG043471 from the National Institute on Aging. Dr. Self was supported in part by K23GM110469 from the National Institute of General Medical Sciences. REDCap, the electronic data capture tool, was supported by UL1 TR000445 from NCATS/NIH.
Footnotes
Meetings: Presented at the Society for Academic Emergency Medicine Conference, New Orleans, LA. May 2016.
Conflicts of Interest and Source of Funding: The authors report no conflicts of interest related to the content of this manuscript.
Author Contributions: CPU and WHS conceived the study. CPU, CGG, and WHS designed the study. CPU, CGG, SR, SPC, MWS, TWR, DL, JME, KH, TWB, CDM, and WHS were involved in data acquisition, analysis, and/or interpretation. CPU and WHS drafted the manuscript, and all authors provided substantial contribution to revision. CPU, CGG, DL, and WHS performed statistical analyses. SR and KH provided administrative or technical support. WHS and SPC supervised the study. WHS takes responsibility for the paper as a whole.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. [Accessed March 23, 2016];Web-based injury statistics query and reporting system (WISQARS) fatal injury data. http://www.cdc.gov/injury/wisqars/fatal.html.
- 2.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. [Accessed March 23, 2016];Web-based injury statistics query and reporting system (WISQARS) nonfatal injury data. http://www.cdc.gov/injury/wisqars/nonfatal.html.
- 3.Mayglothling J, Duane T, Gibbs M, et al. Emergency tracheal intubation immediately following traumatic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5):S333–S340. doi: 10.1097/TA.0b013e31827018a5. [DOI] [PubMed] [Google Scholar]
- 4.Smith DC, Bergen A, Smithline H, Kirschner R. A trial of etomidate for rapid sequence intubation in the emergency department. J Emerg Med. 2000;18(1):13–16. doi: 10.1016/s0736-4679(99)00154-7. [DOI] [PubMed] [Google Scholar]
- 5.Sehdev RS, David SAD, Korana K. Ketamine for rapid sequence induction in patients with head injury in the emergency department. Emerg Med Australas. 2006;18(1):37–44. doi: 10.1111/j.1742-6723.2006.00802.x. [DOI] [PubMed] [Google Scholar]
- 6.Miller RD, editor. Miller’s Anesthesia. New York, NY: Elsevier/Churchill Livingstone; 2005. pp. 346–348. [Google Scholar]
- 7.Craven R. Ketamine. Anaesthesia. 2007;62(s1):48–53. doi: 10.1111/j.1365-2044.2007.05298.x. [DOI] [PubMed] [Google Scholar]
- 8.Bergen JM, Smith DC. A review of etomidate for rapid sequence intubation in the emergency department. J Emerg Med. 1997;15(2):221–230. doi: 10.1016/s0736-4679(96)00350-2. [DOI] [PubMed] [Google Scholar]
- 9.Paris A, Philipp M, Tonner PH, et al. Activation of alpha 2B-adrenoceptors mediates the cardiovascular effects of etomidate. Anesthesiology. 2003;99(4):889–895. doi: 10.1097/00000542-200310000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Joint Theatre Trauma System. [Accessed March 29, 2016];Clinical practice guidelines: trauma airway management. www.usaisr.amedd.army.mil/cpgs.html.
- 11.Duthie DJR, Fraser R, Nimmo WS. Effect of induction of anaesthesia with etomidate on corticosteroid synthesis in man. Br J Anaesth. 1985;57(2):156–159. doi: 10.1093/bja/57.2.156. [DOI] [PubMed] [Google Scholar]
- 12.De Coster R, Helmers JH, Noorduin H. Effect of etomidate on cortisol biosynthesis: site of action after induction of anaesthesia. Acta endocrinol. 1985;110(4):526–531. doi: 10.1530/acta.0.1100526. [DOI] [PubMed] [Google Scholar]
- 13.Diago MC, Amado JA, Otero M, et al. Anti-adrenal action of a subanaesthetic dose of etomidate. Anaesthesia. 1988;43(8):644–645. doi: 10.1111/j.1365-2044.1988.tb04148.x. [DOI] [PubMed] [Google Scholar]
- 14.Vinclair M, Broux C, Faure P, et al. Duration of adrenal inhibition following a single dose of etomidate in critically ill patients. Intensive Care Med. 2008;34(4):714–719. doi: 10.1007/s00134-007-0970-y. [DOI] [PubMed] [Google Scholar]
- 15.Schenarts CL, Burton JH, Richard RR. Adrenocortical dysfunction following etomidate induction in emergency department patients. Acad Emerg Med. 2001;8(1):1–7. doi: 10.1111/j.1553-2712.2001.tb00537.x. [DOI] [PubMed] [Google Scholar]
- 16.Absalom A, Pledger D, Kong A. Adrenocortical function in critically ill patients 24h after a single dose of etomidate. Anaesthesia. 1999;54:861–867. doi: 10.1046/j.1365-2044.1999.01003.x. [DOI] [PubMed] [Google Scholar]
- 17.Pagel PS, Kampine JP, Schmeling JP, et al. Ketamine depresses myocardial contractility as evaluated by the preload recruitable stroke work relationship in chronically instrumented dogs with autonomic nervous system blockade. Anesthesiology. 1992;76(4):564–572. doi: 10.1097/00000542-199204000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Dewhirst E, Frazier WJ, Leder M, et al. Cardiac arrest following ketamine administration for rapid sequence intubation. J Intensive Care Med. 2013;28(6):375–379. doi: 10.1177/0885066612448732. [DOI] [PubMed] [Google Scholar]
- 19.Scherzer D, Leder M, Tobias JD. Pro-con debate: etomidate or ketamine for rapid sequence intubation in pediatric patients. J Pediatr Pharmacol Ther. 2012;17(2):142–149. doi: 10.5863/1551-6776-17.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filanovsky Y, Miller P, Kao J. Myth: ketamine should not be used as an induction agent for intubation in patients with head injury. CJEM. 2010;12(02):154–157. doi: 10.1017/s1481803500012197. [DOI] [PubMed] [Google Scholar]
- 21.Cohen L, Athaide V, Wickham ME, et al. The effect of ketamine on intracranial and cerebral perfusion pressure and health outcomes: a systematic review. Ann Emerg Med. 2015;65(1):43–51. doi: 10.1016/j.annemergmed.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Warner KJ, Cuschieri J, Jurkovich GJ, et al. Single-dose etomidate for rapid sequence intubation may impact outcome after severe injury. J Trauma Acute Care Surg. 2009;67(1):45–50. doi: 10.1097/TA.0b013e3181a92a70. [DOI] [PubMed] [Google Scholar]
- 23.Hildreth A, Mejia VA, Maxwell RA, et al. Adrenal suppression following a single dose of etomidate for rapid sequence induction: a prospective randomized study. J Trauma. 2008;65(3):573. doi: 10.1097/TA.0b013e31818255e8. [DOI] [PubMed] [Google Scholar]
- 24.Cotton BA, Guillamondegui OD, Fleming SB, et al. Increased risk of adrenal insufficiency following etomidate exposure in critically injured patients. Arch Surg. 2008;143(1):62–67. doi: 10.1001/archsurg.143.1.62. [DOI] [PubMed] [Google Scholar]
- 25.Asehnoune K, Mahe PJ, Seguin P, et al. Etomidate increases susceptibility to pneumonia in trauma patients. Intensive Care Med. 2012;38(10):1673–1682. doi: 10.1007/s00134-012-2619-8. [DOI] [PubMed] [Google Scholar]
- 26.Cohan P, Wang C, McArthur DL, et al. Acute secondary adrenal insufficiency after traumatic brain injury: A prospective study*. Crit Care Med. 2005;33(10):2358–2366. doi: 10.1097/01.ccm.0000181735.51183.a7. [DOI] [PubMed] [Google Scholar]
- 27.Bernard F, Outtrim J, Menon DK, et al. Incidence of adrenal insufficiency after severe traumatic brain injury varies according to definition used: clinical implications. Br J Anaesth. 2006;96(1):72–76. doi: 10.1093/bja/aei277. [DOI] [PubMed] [Google Scholar]
- 28.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Kaji AH, Schriger D, Green S. Looking through the retrospectoscope: reducing bias in emergency medicine chart review studies. Ann Emerg Med. 2014;64(3):292. doi: 10.1016/j.annemergmed.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haider AH, Saleem T, Leow JJ, et al. Influence of the National Trauma Data Bank on the study of trauma outcomes: is it time to set research best practices to further enhance its impact? J Am Coll Surg. 2012;214(5):756–768. doi: 10.1016/j.jamcollsurg.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haider AH, Hashmi ZG, Zafar SN, et al. Developing best practices to study trauma outcomes in large databases: an evidence-based approach to determine the best mortality risk adjustment model. J Trauma Acute Care Surg. 2014;76(4):1061–1069. doi: 10.1097/TA.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 33.Harrell FE. With Applications to Linear Models, Logistic Regression, and Survival Analysis. 1. New York, NY: Springer; 2001. Regression Modeling Strategies; p. 23. [Google Scholar]
- 34.Wang R, Lagakos SW, Ware JH, et al. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 35.Matowe LK, Leister CA, Crivera C, et al. Interrupted time series analysis in clinical research. Ann Pharmacother. 2003;37(7–8):1110–1116. doi: 10.1345/aph.1A109. [DOI] [PubMed] [Google Scholar]
- 36.Grijalva CG, Nuorti JP, Arbogast PG, et al. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369(9568):1179–1186. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- 37.Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 38.Albert SG, Ariyan S, Rather A. The effect of etomidate on adrenal function in critical illness: a systematic review. Intensive care med. 2011;37(6):901–910. doi: 10.1007/s00134-011-2160-1. [DOI] [PubMed] [Google Scholar]
- 39.Hohl CM, Kelly-Smith CH, Yeung TC, et al. The effect of a bolus dose of etomidate on cortisol levels, mortality, and health services utilization: a systematic review. Ann emerg med. 2010;56(2):105–113. doi: 10.1016/j.annemergmed.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 40.Malerba G, Romano-Girard F, Cravoisy A, et al. Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation. Intensive care med. 2005;31(3):388–392. doi: 10.1007/s00134-004-2550-8. [DOI] [PubMed] [Google Scholar]
- 41.Brown CA, Bair AE, Pallin DJ, et al. Techniques, success, and adverse events of emergency department adult intubations. Ann Emerg Med. 2015;65(4):363–370. doi: 10.1016/j.annemergmed.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 42.Hinkewich C, Green R. The impact of etomidate on mortality in trauma patients. Can J Anaesth. 2014;61(7):650–655. doi: 10.1007/s12630-014-0161-6. [DOI] [PubMed] [Google Scholar]
- 43.Jabre P, Combes X, Lapostolle F, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009;374(9686):293–300. doi: 10.1016/S0140-6736(09)60949-1. [DOI] [PubMed] [Google Scholar]
- 44.Gelissen HP, Combes X, Laspostolle F, et al. Inotropic effects of propofol, thiopental, midazolam, etomidate, and ketamine on isolated human atrial muscle. Anesthesiology. 1996;84(2):397–403. doi: 10.1097/00000542-199602000-00019. [DOI] [PubMed] [Google Scholar]
- 45.Waxman K, Shoemaker WC, Lippmann M. Cardiovascular effects of anesthetic induction with ketamine. Anesth Analg. 1980;59(5):355–358. [PubMed] [Google Scholar]
- 46.Miller M, Kruit N, Heldreich C, et al. Hemodynamic response after rapid sequence induction with ketamine in out-of-hospital patients at risk of shock as defined by the shock index. Ann emerg med. 2016 doi: 10.1016/j.annemergmed.2016.03.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E1. Survival curves displaying the percentage of patients alive over time by induction agent received. Observation time was censored at hospital discharge. The adjusted hazard ratio (aHR) compares the hazard of death for patients who received ketamine to those who received etomidate (referent). Model covariates included: age, gender, heart rate, systolic blood pressure, Glasgow Coma Scale score, Injury Severity Score, and injury mechanism.
Table E1. Patient characteristics by period in relation to the protocol switch from etomidate to ketamine for ED RSI of trauma patients. The etomidate period included the 11 bimonths between January 2011 and October 2012. The two bimonths between November 2012 and February 2013 were considered a transition period. The ketamine period included the 11 bimonths between March 2013 and December 2014.