Fig. 2.
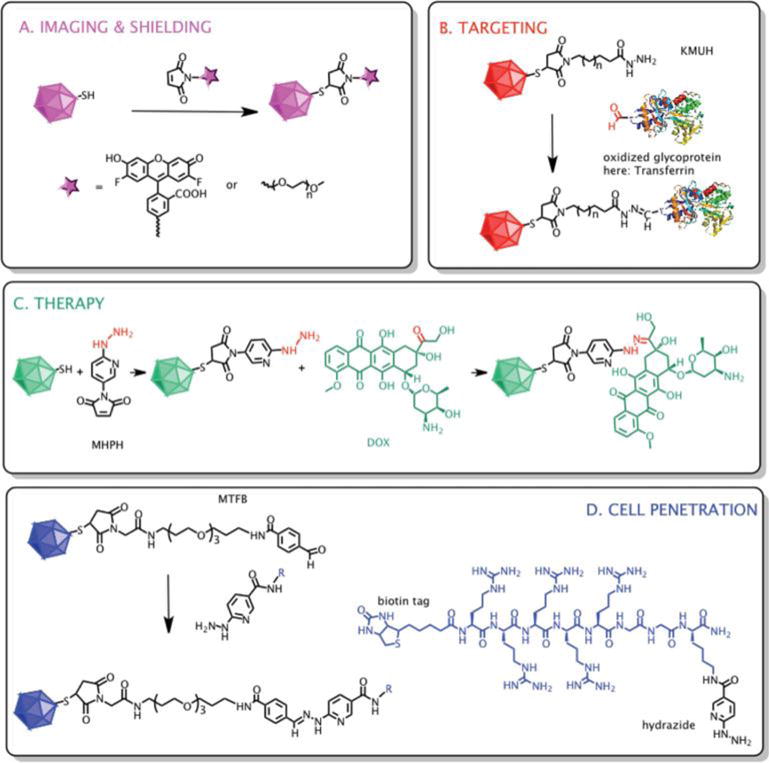
Design and reaction scheme of surface modifications of cBMV with different ligands. (A) cBMV particles were reacted with fluorescent OregonGreen 488 and PEG (MW 2000 Da, n = 44) molecules via maleimide–thiol chemistry. (B) Oxidized transferrin (structure was obtained from pdb.com) was introduced to particles through a two-step reaction by employing first thiol–maleimide then aldehyde–hydrazide coupling using the bivalent linker KMUH (n = 7). (C) Doxorubicin molecules were attached to cBMV particles in a two-step reaction utilizing thiol–maleimide and ketone–hydrazide coupling using the linker MHPH. (D) R5-peptide was linked to particles in a two-step reaction, first MTFB was introduced using maleimide–thiol chemistry, second R5 was conjugated via aldehyde–hydrazide linkage.
