Abstract
OBJECTIVE
The superior effect of Roux-en-Y gastric bypass (RYGB) on glucose control compared with laparoscopic adjustable gastric banding (LAGB) is confounded by the greater weight loss after RYGB. We therefore examined the effect of these two surgeries on metabolic parameters matched on small and large amounts of weight loss.
RESEARCH DESIGN AND METHODS
Severely obese individuals with type 2 diabetes were tested for glucose metabolism, β-cell function, and insulin sensitivity after oral and intravenous glucose stimuli, before and 1 year after RYGB and LAGB, and at 10% and 20% weight loss after each surgery.
RESULTS
RYGB resulted in greater glucagon-like peptide 1 release and incretin effect, compared with LAGB, at any level of weight loss. RYGB decreased glucose levels (120 min and area under the curve for glucose) more than LAGB at 10% weight loss. However, the improvement in glucose metabolism, the rate of diabetes remission and use of diabetes medications, insulin sensitivity, and β-cell function were similar after the two types of surgery after 20% equivalent weight loss.
CONCLUSIONS
Although RYGB retained its unique effect on incretins, the superiority of the effect of RYGB over that of LAGB on glucose metabolism, which is apparent after 10% weight loss, was attenuated after larger weight loss.
Introduction
Surgical weight loss leads to improved glucose control with remission of type 2 diabetes in 30–80% of cases (1,2). Surgeries, such as Roux-en-Y gastric bypass (RYGB), with rerouting of nutrients away from the upper part of the gastrointestinal track, are more successful at controlling type 2 diabetes than purely restrictive surgeries, such as laparoscopic adjustable gastric banding (LAGB) (3). In addition to being more efficient, the metabolic improvements after RYGB appear faster than those after LAGB (4,5), occur after minimal weight loss, and may be mediated by gut-dependent mechanisms, independent of weight change (6,7). However, the superior effect of RYGB on diabetes, compared with LAGB (8,9), is often confounded by greater weight loss after RYGB (3,10–13).
To investigate the contribution of weight loss amount versus altered nutrient route to improvement in β-cell function, we compared the effect of RYGB and LAGB on incretin effect, β-cell glucose sensitivity (BCGS), and insulin sensitivity in individuals with type 2 diabetes before and 1 year after surgery, and/or after 10% and at 20% matched weight loss after the two types of surgery. Furthermore, to identify the role of the incretin effect on glucose and insulin parameters, all subjects were studied after oral and intravenous isoglycemic glucose stimuli. Our primary hypothesis was that the differential effect of the two types of surgery on insulin secretion and β-cell function would be apparent only after an oral glucose challenge, but not after an intravenous glucose challenge. A secondary hypothesis was that changes in insulin sensitivity would track weight loss equally after the two types of surgery.
Research Design and Methods
Subjects
The study was conducted at Mount Sinai St. Luke’s Hospital. Subjects were selected from an eligible pool of severely obese individuals with type 2 diabetes, who were scheduled to undergo either RYGB or LAGB. All subjects provided written informed consent prior to participating. Exclusion criteria included age <21 or >65 years, and BMI <35 or >50 kg/m2, and treatment with dipeptidyl peptidase 4 (DPP-4) inhibitors, thiazolidinediones, or glucagon-like peptide 1 (GLP-1) agonists.
Study Design
This is a longitudinal prospective study of individuals with obesity and type 2 diabetes enrolled in the month prior to their bariatric surgery, and studied at 10% and 20% matched weight loss and/or at 1 year after surgery. Diabetes remission was defined using American Diabetes Association criteria, with HbA1c levels <6.5% (48 mmol/mol), fasting glucose levels <126 mg/dL, and 120 min postprandial glucose levels <200 mg/dL (14).
Interventions
RYGB
Laparoscopic surgery with a 30-mL gastric pouch, a 40-cm afferent limb, a 150-cm Roux limb, and a 12-mm gastrojejunostomy, as described previously (7).
LAGB
A silicone adjustable band (∼10–12 mm diameter) was placed around the proximal portion of the stomach, creating a 30-mL pouch. Adjustment of the band with saline was performed as needed.
Diet for RYGB and LAGB
Subjects were free living, but the recommended postoperative diet is clear liquids during week 1, pureed diet during weeks 1–3, and solid foods starting at week 4.
Experimental Procedures
Oral Glucose Tolerance Test
Participants underwent a 3-h oral glucose tolerance test (OGTT; 50 g of glucose in 200 mL) after a 12-h overnight fast. Blood samples were collected over 3 h from an antecubital intravenous catheter from an arterialized arm vein kept warm with a heating pad, in chilled EDTA tubes; blood samples for incretins were also collected with aprotinin (500 kallikrein inhibitory units/mL blood; Roche Life Science, Indianapolis, IN) and DPP-4 inhibitor (50 µmol/L or 10 µL/mL blood) (EMD Millipore, St. Charles, MO). Samples were centrifuged at 4°C and stored at −80°C.
Isoglycemic Intravenous Glucose Clamp
To measure the incretin effect and to calculate the relative insulin secretion after oral and matched IV glucose, an isoglycemic glucose clamp (iso-IVGC) was performed, as described previously (6). Glucose (sterile 20% dextrose solution) was infused using a Gemini pump (CareFusion, San Diego, CA) over a 3-h time period. Blood glucose levels were monitored using contralateral antecubital intravenous access every 5 min, and the glucose infusion rate was adjusted accordingly, in an effort to mimic the glucose concentration profiles achieved for each patient during the OGTT. Blood samples were collected over 3 h as described above.
Insulin-Modified Frequently Sampled Intravenous Glucose Tolerance Test
An insulin-modified, frequently sampled intravenous glucose tolerance test (IVGTT) was performed before and 1 year after surgery. Glucose (0.3 g/kg body wt as dextrose 50 g/dL) was administered intravenously over ∼1 min, and blood was sampled using a contralateral antecubital vein intravenous cannula at 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, and 19 min thereafter. At 20 min, 0.025 units/kg insulin was injected over 20 s, and blood was sampled at 22, 24, 25, 27, 30, 40, 50, 60, 70, 90, 100, 120, 140, 160, and 180 min (15).
Body Composition
Fat mass was measured using a three-dimensional photonic scanner (Hamamatsu Photonics) (16,17) before and 1 year after surgery.
Assays
Plasma glucose was determined bedside by the glucose oxidase method with a glucose analyzer (Analox, Lunenburg, MA). Total GLP-1 level was measured by radioimmunoassay after plasma ethanol extraction. This assay reacts 100% with GLP-1(17-36), GLP-1(19-36), and GLP-1(17-37), but not with glucagon (0.2%), GLP-2 (<0.01%), or exendin (<0.01%). Gastric inhibitory polypeptide (GIP) was determined by ELISA, which reacts 100% with GIP(1-42) and GIP(3-42), but not with GLP-1, GLP-2, oxyntomodulin, or glucagon. Plasma insulin and C-peptide levels were measured by radioimmunoassay. All hormone assays were performed at the Hormonal Core Laboratory at the New York Obesity Nutrition Research Center with commercial kits (EMD Millipore). Intra-assay and interassay coefficients of variation ranged from 3.4% to 7.4% and from 4.4% to 7.4%, respectively.
Calculations
Total area under the curve (AUC) during the OGTT was calculated using the trapezoidal method. The insulin response to oral and intravenous-isoglycemic glucose clamp were used to calculate the relative incretin effect (%) on insulin and C-peptide levels, as follows:
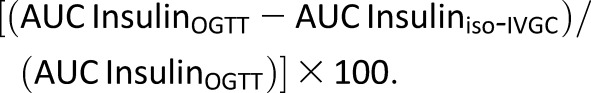 |
The OGTT insulin sensitivity index (ISI) or the Matsuda index calculated as follows:
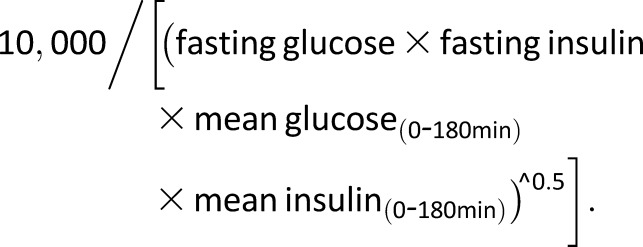 |
HOMA of insulin resistance (HOMA-IR) (18) calculated as:
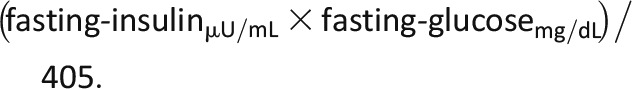 |
Insulin sensitivity was also assessed using the Bergman minimal model analysis of the insulin modified frequently sample IVGTT (15). This model provides equations to measure the acute insulin response to glucose (AIRg; i.e., insulin secretion), the glucose-dependent glucose disappearance (Sg), and the sensitivity of glucose disappearance after insulin (insulin sensitivity [Si]). The intravenous disposition index (DI) was calculated in response to the IVGTT (DIIV (IVGTT)), which is derived from the product of Si and AIRg, as well as in response to the iso-IVGC (DIIV (iso-IVGC)), which is derived from the product of IV-BCGS and 1/HOMA-IR. The insulinogenic index was calculated using ∆Insulin(0–30)(pmol ⋅ L−1)/∆Glucose(0–30)(mmol ⋅ L−1) from the OGTT. The oral DI (DIO (HOMA-IR)) is derived from the product of the insulinogenic index and the inverse of HOMA-IR (19). An additional measure of the DIO (DIO (ISI)) was derived from the product of the insulinogenic index and ISI.
Insulin secretion rates (ISRs) calculated by mathematical deconvolution using a two-compartment model for hormone clearance using C-peptide levels derived from the OGTT (O-ISR) and iso-IVGC (IV-ISR), using the Chronobiological Series Analyzer (Van Cauter, Hasak, and Leproult, University of Chicago, Chicago, IL) (20). BCGS was calculated as the slope between the ISR (pmol ⋅ kg−1 ⋅ min−1) and the corresponding blood glucose level (mmol ⋅ L−1), from baseline to peak glucose level, from OGTT (O-BCGS) and iso-IVGC (IV-BCGS).
Nomenclature
Variables derived from OGTT and iso-IVGC are preceded by “O-” and “IV-,” respectively (e.g., O-BCGS, IV-BCGS) (see Supplementary Table 1).
Statistical Analysis
Normality was tested and variables were log transformed if necessary. Nonparametric tests were used if variables were still not normally distributed. Independent and paired t tests were used for RYGB versus LAGB, and preintervention versus ∼10% weight loss, respectively. Repeated-measures ANOVA was used to compare plasma glucose matching between the OGTT and iso-IVGC. Linear mixed-model regression analysis was used to test the effect of surgery type and percentage of weight loss on outcome variables. Data are expressed as the mean ± SD except in figures where values are reported as the mean ± SEM. Statistical significance was set at P < 0.05 (two-tailed). IBM SPSS version 22.0 was used for all analyses.
Results
Recruitment and Retention
Of the 61 enrolled participants, 41 (26 RYGB participants, 15 LAGB participants) were restudied at 10% weight loss, and 39 (27 RYGB and 12 LAGB) were restudied 1 year postsurgery.
Baseline Characteristics
Age (LAGB group 48.5 ± 10.2 years, RYGB group 43.7 ± 8.2 years), diabetes duration (LAGB group 35.7 ± 36.7 months, RYGB group 29.6 ± 27.2 months), diabetes control (HbA1c level: LAGB group 6.5 ± 0.9% or 48 ± 6.64 mmol/mol; RYGB group 6.8 ± 0.7% or 48 ± 5.16 mmol/mol), use of oral diabetes medications (LAGB group 8 of 12 subjects, RYGB group 20 of 26 subjects) and insulin (LAGB group 0 of 12 subjects, RYGB group 2 of 26 subjects), weight, BMI, fat mass, fasting and postprandial glucose concentrations, insulin sensitivity (HOMA-IR, ISI, and Si by IVGTT), incretin effect, ISR, BCGS after oral or intravenous glucose, AIRg, and DI were similar between groups prior to intervention (Tables 1 and 2 and Supplementary Tables 2–4). Baseline data were also not different between subjects that completed all study visits and those who did not (data not shown).
Table 1.
Metabolic and hormonal changes 1 year after LAGB and RYGB
| LAGB (n = 12) |
RYGB (n = 27) |
P values | |||||
|---|---|---|---|---|---|---|---|
| Presurgery | 1 Year postsurgery | Δ | Presurgery | 1 Year postsurgery | Δ | ||
| BMI (kg ∙ m−2) | 43.4 ± 4.9 | 36.1 ± 5.7 | 8.1 ± 5.1* | 44.6 ± 3.7 | 31.2 ± 3.4# | 13.0 ± 4.3* | 0.010 |
| Weight loss (%) | 16.6 ± 9.8 | 30.1 ± 3.4# | <0.001 | ||||
| Weight loss duration (weeks) | 56.1 ± 17.5 | 49.7 ± 12.2 | 0.655 | ||||
| Glucose infused (Iso-IVGC) (g) | 41.8 ± 20.2 | 30.2 ± 12.5 | 11.0 ± 25.3 | 42.7 ± 12.2 | 34.3 ± 13.1 | 8.1 ± 17.7* | 0.725 |
| Fasting glucose (mmol ∙ L−1) | 7.8 ± 1.9 | 5.6 ± 1.0 | 2.1 ± 3.5* | 7.5 ± 1.8 | 5.1 ± 0.9 | 2.3 ± 1.9* | 0.768 |
| 120-min glucose (mmol ∙ L−1) | 10.7 ± 3.2 | 8.5 ± 2.5 | 1.9 ± 3.5* | 10.8 ± 2.9 | 5.4 ± 1.6# | 5.5 ± 3.4* | 0.005 |
| Glucose AUC (mmol ∙ L−1 ∙ min−1) | 10.6 ± 2.9 | 8.1 ± 1.9 | 2.5 ± 2.8* | 10.5 ± 2.4 | 7.0 ± 1.6 | 3.5 ± 3.0* | 0.336 |
| O-ISR AUC0–180 (pmol ∙ kg−1 ∙ min−1) | 824.4 ± 455.0 | 883.1 ± 527.1† | 38.8 ± 447.3 | 876.9 ± 365.3† | 935.0 ± 470.0† | 97.2 ± 640.4 | 0.460 |
| O-ISR AUC0–60 (pmol ∙ kg−1 ∙ min−1) | 260.6 ± 153.0† | 265.9 ± 150.1† | 27.9 ± 112.6 | 269.4 ± 114.6† | 530.1 ± 281.2†# | 276.9 ± 312.9* | <0.001 |
| IV-ISR AUC0–180 (pmol ∙ kg−1 ∙ min−1) | 728.4 ± 496.1 | 559.4 ± 257.1 | 200.7 ± 443.2 | 758.9 ± 296.7 | 632.9 ± 260.5 | 119.4 ± 373.2* | 0.590 |
| IV-ISR AUC0–60 (pmol ∙ kg−1 ∙ min−1) | 195.9 ± 111.1 | 170.2 ± 79.5 | 26.9 ± 81.9 | 205.6 ± 105.5 | 228.0 ± 100.1 | 20.0 ± 108.3 | 0.154 |
| HOMA-IR | 10.3 ± 7.7 | 3.8 ± 2.8 | 6.8 ± 6.4* | 9.5 ± 4.3 | 2.0 ± 1.1# | 7.1 ± 3.7* | 0.868 |
| ISI | 2.5 ± 1.8 | 5.4 ± 3.5 | 3.1 ± 3.3* | 1.8 ± 0.5 | 6.7 ± 5.0 | 4.8 ± 5.0* | 0.222 |
| O-BCGS (pmol ∙ kg−1 ∙ min−1 ∙ mmol/L−1) | 0.56 ± 0.49 | 1.09 ± 0.65† | 0.53 ± 0.81* | 0.61 ± 0.43† | 1.96 ± 1.16†# | 1.33 ± 1.2* | 0.024 |
| IV-BCGS (pmol ∙ kg−1 ∙ min−1 ∙ mmol/L−1) | 0.38 ± 0.28 | 0.47 ± 0.48 | 0.09 ± 0.55 | 0.35 ± 0.26 | 0.57 ± 0.42 | 0.21 ± 0.49* | 0.541 |
| Sg | 0.01 ± 0.002 | 0.02 ± 0.005 | 0.007 ± 0.004* | 0.01 ± 0.004 | 0.02 ± 0.005 | 0.003 ± 0.005* | 0.110 |
| AIRg (mU ∙ L−1 ∙ min−1) | 92.4 ± 181.4 | 160.3 ± 157.5 | 67.9 ± 102.9 | 122.5 ± 140.5 | 218.3 ± 220.2 | 95.8 ± 215.2 | 0.699 |
| Si (pmol/L) | 1.6 ± 1.7 | 3.5 ± 1.9 | 2.0 ± 1.4* | 1.8 ± 0.8 | 3.2 ± 1.5 | 2.0 ± 1.4* | 0.898 |
| DIIV (IVGTT) | 161.9 ± 499.1 | 558.2 ± 646.9 | 396.3 ± 375.3* | 166.9 ± 210.0 | 654.2 ± 522.6 | 487.3 ± 430.5* | 0.631 |
| DIIV (iso-IVGC) | 0.05 ± 0.05 | 0.21 ± 0.29 | 0.15 ± 0.31 | 0.06 ± 0.07 | 0.46 ± 0.70 | 0.41 ± 0.71* | 0.141 |
| DIO (HOMA-IR) | 16.3 ± 25.2 | 30.3 ± 21.1 | 13.9 ± 22.1 | 10.8 ± 7.4 | 70.2 ± 47.4# | 59.4 ± 47.9* | <0.001 |
| Insulin incretin effect (%) | 24.5 ± 25.1 | 36.0 ± 16.1 | 11.5 ± 25.8 | 20.5 ± 19.5 | 51.4 ± 18.6# | 33.4 ± 29.5* | 0.029 |
Values are reported as the mean ± SD, unless otherwise indicated. Δ, change with intervention. The P values reported are for the difference between change with LAGB and with RYGB.
*P < 0.05 vs. preintervention;
†P < 0.05 vs. intravenous;
#P < 0.05 difference between LAGB and RYGB (n = 27) and LAGB (n = 12) except for body composition and IVGTT (RYGB n = 13, LAGB n = 7).
Table 2.
Comparison of RYGB and LAGB at 10% matched weight loss
| LAGB (n = 15) |
RYGB (n = 26) |
P values | |||||
|---|---|---|---|---|---|---|---|
| Pre-LAGB | 10% Weight loss | Δ | Pre-RYGB | 10% Weight loss | Δ | ||
| BMI (kg/m2) | 42.4 ± 4.9 | 38.1 ± 4.6 | 4.3 ± 1.6* | 44.7 ± 3.6 | 40.3 ± 3.6 | 4.4 ± 1.1* | 0.846 |
| Weight loss (%) | 9.6 ± 2.0 | 10.0 ± 2.0 | 0.633 | ||||
| Weight loss duration (weeks) | 8.7 ± 8.5 | 4.2 ± 0.9 | 0.020 | ||||
| Glucose infused (iso-IVGC) (g) | 44.6 ± 19.1 | 44.7 ± 16.2 | 0.004 ± 19.4 | 40.5 ± 9.4 | 35.6 ± 12.9 | 5.0 ± 16.0 | 0.075 |
| Fasting glucose (mmol ∙ L−1) | 8.1 ± 2.0 | 6.8 ± 1.8 | 1.3 ± 1.6* | 7.4 ± 1.5 | 5.6 ± 0.8# | 1.6 ± 1.5* | 0.574 |
| 120-min glucose (mmol ∙ L−1) | 11.7 ± 3.7 | 11.4 ± 4.4 | 0.3 ± 2.0 | 10.7 ± 2.8 | 6.6 ± 1.9# | 4.1 ± 2.6* | <0.001 |
| Glucose AUC (mmol ∙ L−1 ∙ min−1) | 11.4 ± 3.3 | 10.5 ± 3.4 | 0.9 ± 1.8* | 10.4 ± 2.3 | 7.9 ± 1.6# | 2.5 ± 2.0* | 0.011 |
| O-ISR AUC0–180 (pmol ∙ kg−1 ∙ min−1) | 839.0 ± 431.6 | 933.3 ± 468.8 | 106.3 ± 795.7 | 853.1 ± 358.9† | 959.8 ± 377.1† | 106.7 ± 288.8 | 0.878 |
| O-ISR AUC0–60 (pmol ∙ kg−1 ∙ min−1) | 262.8 ± 144.0† | 287.2 ± 151.8† | 24.4 ± 95.1 | 260.6 ± 113.6† | 459.3 ± 192.6†# | 198.7 ± 129.4* | <0.001 |
| IV-ISR AUC0–180 (pmol ∙ kg−1 ∙ min−1) | 767.3 ± 453.2 | 801.9 ± 368.4 | 34.6 ± 178.9 | 732.1 ± 290.3 | 657.4 ± 237.0 | 74.7 ± 184.7 | 0.327 |
| IV-ISR AUC0–60 (pmol ∙ kg−1 ∙ min−1) | 202.5 ± 100.7 | 218.9 ± 118.3 | 16.4 ± 81.0 | 203.2 ± 105.2 | 226.5 ± 102.0* | 23.3 ± 49.4* | 0.768 |
| HOMA-IR | 9.1 ± 5.2 | 5.7 ± 3.1 | 3.5 ± 4.5* | 9.6 ± 3.9 | 4.0 ± 2.0 | 5.6 ± 3.8* | 0.134 |
| ISI | 2.3 ± 1.6 | 3.1 ± 2.3 | 0.8 ± 2.0* | 1.8 ± 0.8 | 3.0 ± 1.7 | 1.2 ± 1.8* | 0.547 |
| DIIV (iso-IVGC) | 0.04 ± 0.05 | 0.18 ± 0.37 | 0.14 ± 0.33 | 0.05 ± 0.05 | 0.17 ± 0.17 | 0.12 ± 0.15* | 0.812 |
| DIO (HOMA-IR) | 13.6 ± 23.1 | 22.8 ± 29.9 | 9.2 ± 15.9* | 10.4 ± 7.0 | 46.6 ± 35.7# | 36.2 ± 36.4* | 0.002 |
| O-BCGS (pmol ∙ kg−1 ∙ min−1 ∙ mmol/L−1) | 0.50 ± 0.46† | 0.89 ± 0.73† | 0.39 ± 0.85* | 0.62 ± 0.41† | 1.54 ± 0.70†# | 0.92 ± 0.65* | 0.047 |
| IV-BCGS (pmol ∙ kg−1 ∙ min−1 ∙ mmol/L−1) | 0.32 ± 0.28 | 0.56 ± 0.54 | 0.24 ± 0.32* | 0.34 ± 0.25 | 0.49 ± 0.34 | 0.15 ± 0.31* | 0.367 |
| Insulin incretin effect (%) | 19.8 ± 25.5 | 34.0 ± 15.5 | 15.3 ± 26.6* | 20.2 ± 20.0 | 50.8 ± 14.3# | 30.6 ± 27.6* | 0.090 |
Values are reported as the mean ± SD, unless otherwise indicated. The P values reported are for the difference between change with LAGB and with RYGB. Δ, change with intervention.
*P < 0.05 vs. preintervention;
†P < 0.05 vs. intravenous;
#P < 0.05 vs. LAGB.
Study 1: Changes 1 Year After RYGB and LAGB
Twenty-seven RYGB subjects and 12 LAGB subjects completed the 1-year study point after surgery (Table 1). As expected, RYGB resulted in about twice the amount of weight loss at 1 year compared with LAGB (30.1% vs. 16.6%). Despite this difference in weight loss, the usage of diabetes medications decreased significantly and similarly in both groups, and the percentage of patients with diabetes in remission at 1 year was similar in the two surgical groups (88% for the RYGB group and 83% for the LAGB group).
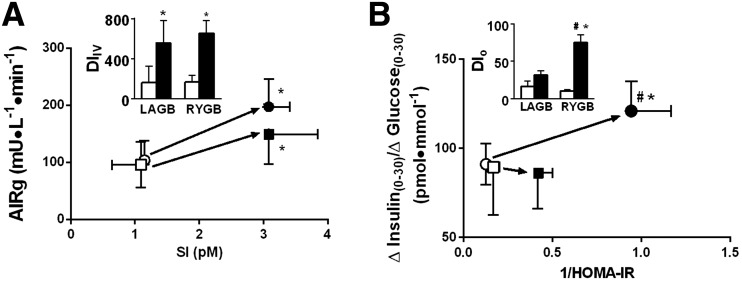
Plasma glucose concentrations were well matched between OGTT and iso-IVGC except after RYGB at any level of weight loss, when the drop in postprandial glucose levels at 90 and 120 min during the OGTT could not be matched by the glucose clamp (Supplementary Fig. 1A and B). As shown before, despite matched glucose levels, the insulin level (AUC) was significantly lower during the iso-IVGC (Supplementary Fig. 1A and B). The amount of glucose delivered during the iso-IVGC was ∼80% of the oral glucose load (50 g) prior to the interventions, and it was only ∼60% 1 year after RYGB and LAGB. RYGB resulted in greater incretin effect (P = 0.016) and GLP-1 release (P = 0.001) (Supplementary Figs. 1C and D, and 2A), better early (0–60 min) β-cell response to oral glucose (P = 0.006) (O-BCGS and O-ISR), lower 120-min glucose level after oral glucose (P = 0.002), and greater improvement in DIO (HOMA-IR) (P = 0.001) compared with LAGB (Table 1, Figs. 1B and 2B, and Supplementary Figs. 1E and F, 2A, and 3). Despite the larger weight loss after RYGB, the improvements in AIRg, SI, DIIV, and fat mass were not significantly different after the two types of surgery (Table 1, Figs. 1A and 2A, and Supplementary Table 2).
Figure 1.
Effect of RYGB and LAGB on the DI in response to IVGTT (A) (n = 7 for LAGB, n = 13 for RYGB); and OGTT (B) (n = 12 for LAGB, n = 27 for RYGB). Insets: Effect of RYGB and LAGB on DIIV (A) and DIO (B). Circles, RYGB; squares, LAGB; open symbols, presurgery; closed symbols, 1 year after surgery. Values are reported as mean ± SEM for all groups. *P < 0.05 vs. preintervention; #P < 0.05 vs. LAGB.
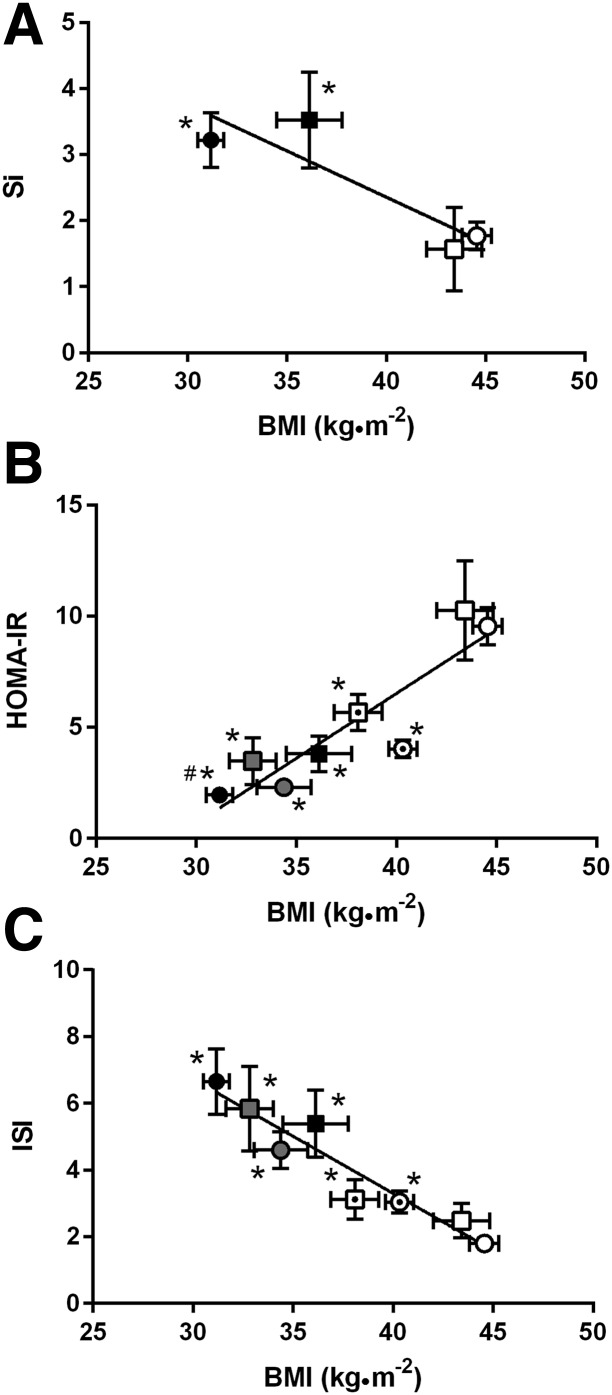
Figure 2.
Relationship between weight loss and improved insulin sensitivity measured during IVGTT (A; LAGB, n = 7; RYGB, n = 13), fasted condition (B), and OGTT (C). Circles, RYGB; squares, LAGB; open symbols, presurgery (LAGB, n = 15; RYGB, n = 27); “target” symbols, 10% matched weight loss (LAGB, n = 15; RYGB, n = 26); gray symbols, 20% matched weight loss (n = 8 for LAGB and RYGB); closed symbols, 1 year after LAGB (n = 12) or RYGB (n = 27). Values are reported as the mean ± SEM for all groups. *P < 0.05 vs. preintervention; #P < 0.05 vs. LAGB.
Study 2a: Comparison of RYGB and LAGB at 10% Matched Weight Loss
Most subjects were restudied at 10% weight loss (LAGB group, n = 15: 9.6 ± 2.0% weight loss; vs. RYGB group, n = 26: 10.0 ± 2.0% weight loss, P = NS). It took twice as long for LAGB patients to lose the same amount of weight (RYGB group 4.2 ± 0.9 weeks, LAGB group 8.7 ± 8.5 weeks, P = 0.020) (Table 2). The improvement in fasting and postprandial glucose levels, GLP-1 release and incretin effect, DIO (HOMA-IR), O-BCGS, and O-ISR was superior after RYGB compared with after LAGB. However, the response to intravenous glucose (IV-BCGS and IV-ISR) and insulin sensitivity (HOMA-IR or ISI) improved similarly in the two surgical groups (Table 2, Fig. 2B and C, and Supplementary Table 3).
Study 2b: Comparison of RYGB and LAGB at 10% and 20% Matched Weight Loss
Only eight individuals in each surgical group were available for study at 20% weight loss (Supplementary Table 4). The time to achieve the 20% weight loss goal was again shorter after RYGB (Supplementary Table 4) (median time 26.2 weeks, range 21.3–109.4 weeks) compared with after LAGB (median time 54.5 weeks, range 52.0–106.7 weeks). Differences in 120-min glucose levels and AUC for glucose observed between groups after 10% matched weight loss largely disappeared after 20% weight loss; insulin sensitivity improved similarly after both types of surgery. However, after RYGB individuals continued to show greater improvement in O-BCGS, incretin effect, and DIO (HOMA-IR) compared with after LAGB (Supplementary Table 4). Although this group was small, data obtained after 10% weight loss did not differ from that obtained for the larger group after the same amount of weight loss.
Weight Loss Versus Surgery Type Effect
Finally, there was a strong relationship shown between insulin sensitivity and BMI (Fig. 2), and between O-BCGS and 2-h plasma glucose level (overall: r = −0.647, P < 0.001; within RYGB: r = −0.652, P < 0.001; within LAGB: r = −0.595, P < 0.001) (Supplementary Fig. 3A). Results from mixed-model regression analysis showed that surgery type and weight loss were both significant predictors of postsurgery glucose level (fasting glucose vs. surgery P = 0.043; weight loss P < 0.001; 120-min glucose vs. surgery P < 0.001; weight loss P < 0.001; glucose AUC vs. surgery P = 0.005; weight loss P < 0.001), β-cell function (DIO (HOMA-IR)) (surgery P = 0.024, weight loss P < 0.001), and O-BCGS (surgery P = 0.018, weight loss P = 0.007). Weight loss, but not surgery type, predicted ISI (P < 0.001), HOMA-IR (P < 0.001), and early-phase insulin secretion (INSAUC0–60) (P < 0.001). Surgery type, but not weight loss, predicted GLP-1 release (GLP-1 peak P < 0.001, GLP-1 AUC P < 0.001) and the incretin effect (incretin effect on insulin P < 0.001, incretin effect on C-peptide level P = 0.033) (Supplementary Table 5).
Conclusions
The major findings of the current study are that in morbidly obese individuals with type 2 diabetes: 1) insulin sensitivity (ISI, HOMA-IR, and Si) and insulin secretory response to an IV stimulus (either AIRg or total ISR from 0 to 180 min during intravenous-isoglycemic clamp) improved as a function of weight loss, regardless of the type of bariatric surgery; 2) BCGS and ISR after oral glucose increased twofold to fourfold more after RYGB compared with after LAGB, regardless of the degree of weight loss; 3) the incretin effect is associated with elevated GLP-1 level and lower postprandial glucose level, an effect specific to RYGB, independent of the degree of weight loss; and 4) the difference in metabolic outcomes between the two types of surgery observed at 10% weight loss is significantly attenuated at 20% weight loss.
Weight loss, but not surgery type, was a predictor of insulin sensitivity by HOMA-IR and ISI. Insulin sensitivity, assessed by HOMA-IR, Si from IVGTT and/or ISI during OGTT, improved after both types of surgery, confirming well-documented data after RYGB (21,22) or LAGB (23–25). Moreover, we show that the effect is a function of weight loss, independent of the surgery type. Insulin sensitivity improves equally after LAGB and RYGB after similar amounts of weight loss. Similar improvement in insulin sensitivity was also shown after 20% matched weight loss after RYGB and LAGB in individuals without diabetes (26), or after 7–8% weight loss by either a very low-calorie diet or RYGB (27).
Similarly, insulin secretion measured after acute (AIRg) or prolonged (3-h intravenous-isoglycemic clamp) intravenous administration of glucose, improves similarly 1 year after RYGB and LAGB. Others have shown recovery of AIRg in individuals with type 2 diabetes as early as 1 month after RYGB with sustained elevation up to 2 years after RYGB (28). Parameters of IVGTT were shown to improve similarly after 7–8% weight loss via either RYGB or a very low-calorie diet in subjects with type 2 diabetes (27).
On the contrary, the greater magnitude of change in parameters of β-cell function (O-BCGS and early-phase insulin secretion or ISR from 0 to 60 min) in response to oral glucose administration after RYGB, compared with LAGB, observed at any level of weight loss, suggest a specific RYGB effect, independent of weight loss. This is in agreement with our working hypothesis. This specific effect of RYGB, not observed after LAGB, is likely related to the unique rise of GLP-1 after RYGB, and can be blocked by the GLP-1 receptor antagonist exendin 9 (29,30). Our data are in agreement with those of Kashyap et al. (31), who showed a greater improvement of β-cell function, assessed during a meal test, 1 week and 4 weeks after RYGB compared with restrictive gastric surgery (vertical sleeve gastrectomy and LAGB) in obese subjects with type 2 diabetes. Both our study and that by Kashyap et al. (31) point to a potential role for the gastrointestinal tract, in addition to weight loss, in the improvement in β-cell function after RYGB during nutrient ingestion. Comparison of the contribution of weight loss versus the gastrointestinal tract in the improvement in β-cell function has been explored by other studies. Salinari et al. (22) compared the effects of the oral versus the IV route on β-cell function and showed that RYGB increased DI during both the OGTT and IVGTT after 10% weight loss in subjects with type 2 diabetes. Although Salinari et al. (22) did not report whether the relative improvement was greater after oral or IV glucose stimulation, it appears that the percentage increase was greater during the OGTT. However, caution should be used for the interpretation of data on β-cell function measured during an OGTT or a meal test after RYGB, because the absorption of glucose is rapid after RYGB.
This is in agreement with our work showing that O-BCGS or O-ISR are 2.5–4.3 larger after RYGB vs LAGB, at any given level of weight loss, while β-cell response to intravenous glucose administration (IV-BCGS and IV-ISR) seems to track weight loss similarly after both types of surgery. The specificity of our study design, using both the oral and IV route to expose the β-cell to matched glycemic stimuli, as well as different levels of weight loss after RYGB and LAGB, gives a unique opportunity to isolate a “gut incretin effect” versus a weight loss effect.
Our group has shown an early (1 month) (6) and durable (3 years) (32) recovery of the blunted incretin effect in patients with type 2 diabetes after RYGB. This effect was not seen after an equivalent 10% weight loss induced by diet (7). Here we confirmed that surgery type, but not weight loss, is the main predictor of the recovery of the incretin effect. The enhanced GLP-1 response is specifically observed after RYGB, at any level of weight loss. We previously reported that GLP-1, which rises consistently after this RYGB (6,7,33,34), was a significant predictor of postsurgery β-cell function, in a cohort observed up to 3 years after RYGB (32). Other studies (35) have also shown that postprandial GLP-1 level is associated with β-cell function in a postbariatric population. Although weight loss had no predictive value on incretin effect in a mixed-model regression analysis, interestingly there was a small, nonsignificant 50–150% increase in the incretin effect after LAGB, albeit of much smaller magnitude than the ∼250–330% increase after RYGB. Because GLP-1 levels do not change after LAGB, GLP-1 is likely not the mediator of the small increase in incretin effect after LAGB, although we cannot exclude a greater sensitivity to endogenous GLP-1 after LAGB weight loss. Recent data (36) show that the density of both cells immunoreactive for GLP-1 and for GIP increased in patients after RYGB. However, the role of GIP as a mediator of the enhanced incretin effect and improved β-cell function after RYGB or LAGB is less well defined, mainly because of the lack of a specific inhibitor available for human testing. A nonspecific amplification of GIP signaling with DPP-4 inhibition did not modify glucose tolerance after RYGB (37). The postprandial change in GIP levels is less consistent after RYGB (6,38) and is of a lesser magnitude than the change in GLP-1 levels. In this study, the greater rise in GIP levels after RYGB, compared with LAGB, was apparent after 10% weight loss. However, after larger weight loss and/or 1 year after both types of surgery, GIP levels were not different between them, suggesting that the new metabolic status of reduced weight, rather than the surgery type, mediated the GIP levels. Therefore, it is possible that some of the recovery of the incretin effect after either type of surgery could be mediated by changes in GIP.
Finally, our data show that the difference in the metabolic outcome between the two types of surgery is attenuated after larger weight loss. This is an important finding. Our study is the only one, to our knowledge, that compares RYGB and LAGB after large weight loss in individuals with type 2 diabetes, matched prior to the surgical interventions for β-cell function and insulin sensitivity. Larger (16.6% or 20%) weight loss after LAGB results in a decrease in fasting glucose levels and glucose AUC similar to that observed after 20% or 30% weight loss after RYGB, showing the importance of achieving a certain amount of weight loss over that of surgery type. This is in agreement with the study by Bradley et al. (26) in individuals without diabetes and with recent data from the Swedish Obese Subjects study (39), which also suggest that weight change, rather than surgery type, is the best predictor of glucose control 2 and 10 years after bariatric surgery.
Therefore, although the magnitude of the weight-independent effect of RYGB on the incretin system during the ingestion of nutrients is highly significant and sustained overtime, its contribution to long-term glucose control and diabetes remission may be limited. These data differ from those reported by Purnell et al. (40), which suggest that the significant differences in 3-year rates of diabetes remission between RYGB and LAGB are independent of weight loss and manifest from unique metabolic mechanisms of RYGB. Their findings may be attributed to possible presurgery differences in β-cell function and insulin sensitivity between their groups, key predictors of glucose control after bariatric surgery, which, unfortunately, were not measured in their study (40).
Although this study makes a critical contribution to the literature, there are several limitations that should be recognized. The assignment to LAGB or RYGB was not randomized, but was dictated by the preferences of patients and surgeons. However, the two groups did not differ before intervention in terms of the following known clinical predictors of diabetes remission: diabetes duration and control, medication and insulin use, and various measures of β-cell function or BMI. Diet was not controlled for, and participants followed the standard dietary recommendation after bariatric surgery. The time it took the LAGB group to achieve any weight loss goals was not only significantly longer, but also highly variable, compared with a shorter and less variable time for the RYGB group. It is likely that the rate of weight loss was faster after RYGB than after LAGB. However, controlling for the rate of weight loss showed equivalent improvement in β-cell function after intravenous glucose administration after RYGB or diet (27), suggesting that this may not be important. The limitations of the techniques used to assess β-cell function in this study, including the 50-g OGTT (vs. 75-g OGTT) and iso-IVGC (to derive β-cell function), with the difficulty in matching glucose levels after RYGB, have been discussed in detail in a recent publication from our group (32). In addition, caution should be used for the interpretation of measures of β-cell function after oral glucose administration due to the change in glucose absorption after RYGB. Follow-up was of short duration (12 months). Finally, the overall number of LAGB participants who completed all study visits was small, especially those who achieved 20% weight loss, which may have underpowered some of the results. Further, this selected group of LAGB participants with large weight loss is likely not representative of the overall effect of LAGB, which is usually more modest. Our study design aimed at using LAGB as a comparative group to RYGB, at a matched 20% weight loss. Challenges included the significant bias of the bariatric surgeons to preferentially offer RYGB—or vertical sleeve gastrectomy—to patients with type 2 diabetes, rather than LAGB, and the highly variable weight loss outcome after LAGB. Despite these difficulties, we were able to show that, after 15–20% weight loss after LAGB, fewer metabolic differences exist between the two types of surgeries, highlighting the importance of the effect of weight loss, in selected groups well matched for preintervention β-cell function, on improvement of glucose metabolism. Our data also confirm the engagement of the gastrointestinal track during nutrient ingestion after RYGB, which provides an additional benefit on β-cell function, via the incretin effect, independent of weight loss. However, the overall importance of the gut mechanism on long-term diabetes remission may be limited.
Supplementary Material
Article Information
Acknowledgments. The authors thank the participants and Fatima Rimawi, Carolina Espinosa, and Daniel Baron Brenner (New York Obesity Nutrition Research Center, Department of Medicine, Columbia University College of Physicians and Surgeons), who assisted in some of the experiments.
Funding. The study was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (grants R01-DK-067561, P30-DK-26687-30, and P30-DK-063608), the American Diabetes Association (grant ADA 1-09-CR-34), and Columbia University (Clinical and Translational Science Award UL1-TR-000040). R.D. was supported by the New York Obesity Research Center training grant 5T32DK007559-22. This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1-TR-000040.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.M.H. helped with data analysis and the writing of the manuscript. R.D. collected and analyzed some of the data and wrote some parts of the manuscript. S.M.S. collected and analyzed some of the data. R.L.P. analyzed the data derived from the intravenous glucose tolerance test and edited the manuscript. P.H. provided statistical consultation and edited the manuscript. J.J.M., S.J.B., C.J.R., and D.R. referred subjects and edited the manuscript. B.L. designed the study, collected and analyzed the data, and wrote the manuscript. B.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-1376/-/DC1.
References
- 1.Schauer PR, Bhatt DL, Kirwan JP, et al.; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med 2014;370:2002–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mingrone G, Panunzi S, De Gaetano A, et al. . Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 3.Courcoulas AP, Belle SH, Neiberg RH, et al. . Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015;150:931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM, et al. . Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jørgensen NB, Jacobsen SH, Dirksen C, et al. . Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab 2012;303:E122–E131 [DOI] [PubMed] [Google Scholar]
- 6.Laferrère B, Heshka S, Wang K, et al. . Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007;30:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laferrère B, Teixeira J, McGinty J, et al. . Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pournaras DJ, Osborne A, Hawkins SC, et al. . Remission of type 2 diabetes after gastric bypass and banding: mechanisms and 2 year outcomes. Ann Surg 2010;252:966–971 [DOI] [PubMed] [Google Scholar]
- 9.Campos GM, Rabl C, Roll GR, et al. . Better weight loss, resolution of diabetes, and quality of life for laparoscopic gastric bypass vs banding: results of a 2-cohort pair-matched study. Arch Surg 2011;146:149–155 [DOI] [PubMed] [Google Scholar]
- 10.Puzziferri N, Nakonezny PA, Livingston EH, Carmody TJ, Provost DA, Rush AJ. Variations of weight loss following gastric bypass and gastric band. Ann Surg 2008;248:233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courcoulas AP, Christian NJ, Belle SH, et al.; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium . Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013;310:2416–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofso D, Jenssen T, Bollerslev J, Ueland T, Godang K, Stumvoll M, Sandbu R, Roislien J, Hjelmesaeth J. Beta cell function after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol 2011;164:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashyap SR, Bhatt DL, Wolski K, et al. . Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care 2013;36:2175–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association Standards of medical care in diabetes-2016 abridged for primary care providers. Clin Diabetes 2016;34:3–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667–E677 [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Gallagher D, Thornton JC, et al. . Regional body volumes, BMI, waist circumference, and percentage fat in severely obese adults. Obesity (Silver Spring) 2007;15:2688–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Gallagher D, Thornton JC, Yu W, Horlick M, Pi-Sunyer FX. Validation of a 3-dimensional photonic scanner for the measurement of body volumes, dimensions, and percentage body fat. Am J Clin Nutr 2006;83:809–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 19.Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE; American Diabetes Association GENNID Study Group . Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes 2002;51:2170–2178 [DOI] [PubMed] [Google Scholar]
- 20.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 21.Martinussen C, Bojsen-Moller KN, Dirksen C, Jacobsen SH, Jørgensen NB, Kristiansen VB, Holst JJ, Madsbad S. Immediate enhancement of first-phase insulin secretion and unchanged glucose effectiveness in patients with type 2 diabetes after Roux-en-Y gastric bypass. Am J Physiol Endocrinol Metab 2015;308:E535–E544 [DOI] [PubMed] [Google Scholar]
- 22.Salinari S, Bertuzzi A, Guidone C, Previti E, Rubino F, Mingrone G. Insulin sensitivity and secretion changes after gastric bypass in normotolerant and diabetic obese subjects. Ann Surg 2013;257:462–468 [DOI] [PubMed] [Google Scholar]
- 23.Zakaria AS, Rossetti L, Cristina M, et al. . Effects of gastric banding on glucose tolerance, cardiovascular and renal function, and diabetic complications: a 13-year study of the morbidly obese. Surg Obes Relat Dis 2016;12:587–595 [DOI] [PubMed] [Google Scholar]
- 24.Samaras K, Viardot A, Botelho NK, Jenkins A, Lord RV. Immune cell-mediated inflammation and the early improvements in glucose metabolism after gastric banding surgery. Diabetologia 2013;56:2564–2572 [DOI] [PubMed] [Google Scholar]
- 25.Urbanavičius V, Abalikšta T, Brimas G, Abraitienė A, Gogelienė L, Strupas K. Comparison of changes in blood glucose, insulin resistance indices, and adipokine levels in diabetic and nondiabetic subjects with morbid obesity after laparoscopic adjustable gastric banding. Medicina (Kaunas) 2013;49:9–14 [PubMed] [Google Scholar]
- 26.Bradley D, Conte C, Mittendorfer B, et al. . Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest 2012;122:4667–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackness C, Karmally W, Febres G, et al. . Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell function in type 2 diabetic patients. Diabetes 2013;62:3027–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin E, Liang Z, Frediani J, et al. . Improvement in ß-cell function in patients with normal and hyperglycemia following Roux-en-Y gastric bypass surgery. Am J Physiol Endocrinol Metab 2010;299:E706–E712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. . Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes 2013;62:3044–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah M, Law JH, Micheletto F, et al. . Contribution of endogenous glucagon-like peptide 1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes 2014;63:483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashyap SR, Daud S, Kelly KR, et al. . Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes 2010;34:462–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dutia R, Brakoniecki K, Bunker P, et al. . Limited recovery of β-cell function after gastric bypass despite clinical diabetes remission. Diabetes 2014;63:1214–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bose M, Teixeira J, Olivan B, et al. . Weight loss and incretin responsiveness improve glucose control independently after gastric bypass surgery. J Diabetes 2010;2:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morínigo R, Moizé V, Musri M, et al. . Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 2006;91:1735–1740 [DOI] [PubMed] [Google Scholar]
- 35.Nannipieri M, Baldi S, Mari A, et al. . Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab 2013;98:4391–4399 [DOI] [PubMed] [Google Scholar]
- 36.Rhee NA, Wahlgren CD, Pedersen J, et al. . Effect of Roux-en-Y gastric bypass on the distribution and hormone expression of small-intestinal enteroendocrine cells in obese patients with type 2 diabetes. Diabetologia 2015;58:2254–2258 [DOI] [PubMed] [Google Scholar]
- 37.Svane MS, Bojsen-Møller KN, Nielsen S, et al. Effects of endogenous GLP-1 and GIP on glucose tolerance after Roux-en-Y gastric bypass surgery. Am J Physiol Endocrinol Metab 2016;310:E505–514 [DOI] [PubMed]
- 38.Jacobsen SH, Olesen SC, Dirksen C, et al. . Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg 2012;22:1084–1096 [DOI] [PubMed] [Google Scholar]
- 39.Sjöholm K, Sjöström E, Carlsson LM, Peltonen M. Weight change-adjusted effects of gastric bypass surgery on glucose metabolism: 2- and 10-year results from the Swedish Obese Subjects (SOS) Study. Diabetes Care 2015;39:625–631 [DOI] [PubMed] [Google Scholar]
- 40.Purnell JQ, Selzer F, Wahed AS, et al. . Type 2 diabetes remission rates after laparoscopic gastric bypass and gastric banding: results of the Longitudinal Assessment of Bariatric Surgery Study. Diabetes Care 2016;39:1101–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.