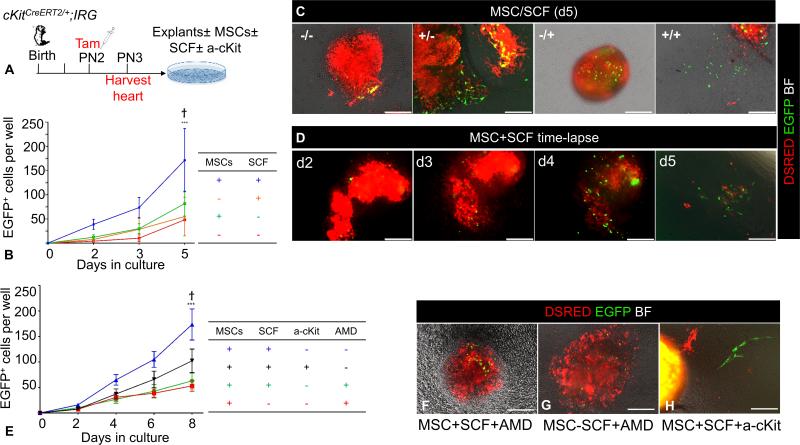
Figure 6. Regulation of CSCs self-renewal by MSCs.
A. Schematic of the ex-vivo genetic fate-mapping strategy to assess the role of MSCs in modulating self-renewal of CSCs (n=6 cKitCreERT2/+;IRG neonates). B, Comparison of the impact of MSC feeders and/or SCF, in the growth of EGFP+ cells after 5 days of cardiac explant culture. C, Representative photomicrographs of day-5 myocardial explant cultures in the presence or absence of MSCs and SCF. Note that, establishment of primary, explant-free, proliferating EGFP+ colonies, requires the presence of both an MSCs feeder layer and exogenous administration of SCF. D, Time-lapse live tissue epifluorescent image of a myocardial explant cultured on an MSC feeder layer, in the presence of exogenously administered SCF. Note the time-dependent proliferative expansion of EGFP+ cells, and eventually their outgrowth and establishment of explant-free colonies within 5 days of tissue culture. E, Impact of SCF/cKit and SDF1/CXCR4 signaling neutralization with an anti-cKit (a-cKit) antibody and AMD3100 respectively (n=2 neonates; 3 myocardial explants/neonate/time point). F-H, Representative photomicrographs of day-6 myocardial explants, cultured on MSCs feeders, in the presence or absence of SCF. Modulating the activity of the SCF/cKit signaling pathway by adding (F) or withdrawing (G) SCF does not rescue the migratory activity of CSCs, following AMD3100 antagonism (n=2 neonates; 3 myocardial explants/neonate/time point). H, Neutralization of the SCF/cKit signaling pathway does not affect EGFP+ cell migration (n=2 neonates; 3 myocardial explants/neonate/time point). AMD, AMD3100. Data are presented as mean±SEM. ***p≤0.0001 within groups. Ϯp=0.001 between groups. Scale bars, 150μm.