Abstract
Objectives
Increasing the ceftaroline fosamil dose beyond 600 mg every 12 h may provide additional benefit for patients with complicated skin and soft tissue infections (cSSTIs) with severe inflammation and/or reduced pathogen susceptibility. A Phase III multicentre, randomized trial evaluated the safety and efficacy of ceftaroline fosamil 600 mg every 8 h in this setting.
Methods
Adult patients with cSSTI and systemic inflammation or comorbidities were randomized 2:1 to intravenous ceftaroline fosamil (600 mg every 8 h) or vancomycin (15 mg/kg every 12 h) plus aztreonam (1 g every 8 h) for 5–14 days. Clinical cure was assessed at the test of cure (TOC) visit (8–15 days after the final dose) in the modified ITT (MITT) and clinically evaluable (CE) populations. Non-inferiority was defined as a lower limit of the 95% CI around the treatment difference greater than –10%. An MRSA-focused expansion period was initiated after completion of the main study. Clinicaltrials.gov registration numbers NCT01499277 and NCT02202135.
Results
Clinical cure rates at TOC demonstrated non-inferiority of ceftaroline fosamil 600 mg every 8 h versus vancomycin plus aztreonam in the MITT and CE populations: 396/506 (78.3%) versus 202/255 (79.2%) patients (difference −1.0%, 95% CI −6.9, 5.4) and 342/395 (86.6%) versus 180/211 (85.3%) patients (difference 1.3%, 95% CI −4.3, 7.5), respectively. In the expansion period, 3/4 (75%) patients treated with ceftaroline fosamil were cured at TOC. The frequency of adverse events was similar between groups.
Conclusions
Ceftaroline fosamil 600 mg every 8 h was effective for cSSTI patients with evidence of systemic inflammation and/or comorbidities. No new safety signals were identified.
Introduction
Complicated skin and soft tissue infections (cSSTIs) represent a common cause of hospitalization.1–3 Due to the increasing prevalence of antibiotic resistance among both Gram-positive and Gram-negative bacterial pathogens, cSSTIs are becoming increasingly challenging to treat.4 Ceftaroline fosamil is approved in the EU for the treatment of patients with community-acquired pneumonia (CAP) and cSSTI,5 and for similar indications elsewhere.
Approvals of the adult dose of ceftaroline fosamil for cSSTI [600 mg every 12 h, administered by 1 h intravenous (iv) infusion] were based on the Phase III CANVAS 1 and 2 trials (NCT00424190 and NCT00423657).2,3 In the individual trials and a pooled analysis, ceftaroline fosamil 600 mg every 12 h was non-inferior to vancomycin plus aztreonam, and clinical cure rates at test of cure (TOC) were similar between treatment groups in both the clinically evaluable (CE) population (91%–93%) and the modified ITT (MITT) population (85%–87%).2,3,6 Ceftaroline fosamil 600 mg every 12 h was generally well tolerated, with a safety profile consistent with the cephalosporin class.2,3,7
Surveillance data indicate that in some Latin American, European and Asian countries, the MIC90 of ceftaroline for MRSA is 2 mg/L;8–10 based on current CLSI and EUCAST susceptibility breakpoints, such strains are considered non-susceptible to ceftaroline fosamil 600 mg every 12 h.11,12 Moreover, severe systemic inflammation can alter antibiotic pharmacokinetics (PK), potentially compromising efficacy.13 An increased dose of ceftaroline fosamil might provide additional benefit for such patients.
The CeftarOline versus Vancomycin and aztrEonam tReating cSSTI (COVERS) trial evaluated the safety and efficacy of ceftaroline fosamil 600 mg every 8 h by 2 h iv infusion in patients with cSSTI and extensive cutaneous involvement, including evidence of systemic inflammation or underlying comorbidities associated with impaired immune response.
Patients and methods
Ethics
The protocol was approved by each study site's independent ethics committees/institutional review board, and the study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization/Good Clinical Practice. All patients provided written informed consent.
Study design and participants
COVERS was a Phase III, prospective, multicentre, randomized, double-blind, non-inferiority trial conducted at 111 centres in 28 countries between May 2012 and June 2014. The complete inclusion/exclusion criteria are available in the Supplementary data (available at JAC Online). Key inclusion criteria: (i) age ≥18 years; (ii) cSSTI [defined as extensive cellulitis, major cutaneous abscess (limited to 30% of the population), burn infection, or traumatic/surgical wound infection with purulent drainage with a minimum surface area of 75 cm2] of sufficient severity to warrant hospitalization and ≥5 days of parenteral antibacterial therapy; and (iii) one or more signs of systemic inflammatory response [body temperature ≥38.0 or ≤36.0°C, white blood cell count >12 000 or <4000 cells/mm3 or >10% band forms (immature white blood cells), heart rate >90 beats/min and respiratory rate >20 breaths/min after 10 min rest], and/or underlying comorbidities associated with impaired immune response.
Key exclusion criteria: uncomplicated skin and soft tissue infections; diabetic foot infections, decubitus ulcers, ulcers due to peripheral vascular disease, necrotizing skin infection or sternal wound infections; or body weight >130 kg.
Randomization and masking
Enrolled patients were assigned a unique randomization code using a centralized telephone/web-based interactive system. The randomization sequence and treatment assignment were generated by a validated computer-generated randomization schedule using a block size of six. Patients were randomized 2:1 to receive ceftaroline fosamil (600 mg every 8 h ) or vancomycin (weight-based dosing) plus aztreonam (1 g every 8 h ) for 5–14 days. Patients and investigators were masked to treatment allocation until study completion.
Procedures
All study treatments were administered iv for 5–14 days, ceftaroline fosamil and vancomycin as 250 mL 2 h infusions and aztreonam as 100 mL 30 min infusions. Dosages were adjusted according to respective product labelling and institutional practice guidelines (see Supplementary data for further details).
Aztreonam could be discontinued at investigators' discretion after ≥3 days if no Gram-negative bacteria were identified by microscopy or culture. To maintain blinding, patients also received matching placebo infusions with volume and infusion duration equivalent to the comparator treatments.
Clinical assessments were conducted daily during the treatment period until the end of treatment (EOT) evaluation (within 24 h of the last dose of study treatment), at the TOC visit (8–15 days after the last dose of study treatment) and at the late follow-up (LFU) visit (21–35 days after the last dose of study treatment).
Assessments included evaluation of the signs and symptoms of cSSTI, physical and laboratory examinations, skin infection site and blood collection for bacterial culture, and recording of adverse events (AEs). Clinical cure was defined as resolution of signs and symptoms of skin infection or improvement of signs and symptoms to such an extent that no further antimicrobial therapy was necessary. Definitions of clinical failure and indeterminate clinical outcomes are available as Supplementary data.
Patients considered clinically cured at TOC were assessed at LFU for evidence of relapse (defined as a relapse of signs and symptoms of cSSTI that required additional antimicrobial therapy) and recurrence and reinfection (defined as isolation of a baseline or new pathogen, respectively, from the original cSSTI site at the LFU visit).
Specimens for microbiological analysis were obtained from the primary infection site at baseline, and after baseline (EOT, TOC and LFU visits) as medically indicated. Specimens were obtained and processed according to recognized methods.14,15
Blood samples for microbiological culture were obtained at baseline, and, for patients with a positive result, repeated daily until negative. Local laboratories and a central reference laboratory (Covance Central Laboratory Services, IN, USA) performed the microbiological assessments. All isolates were shipped to the central laboratory for confirmation of genus/species and susceptibility testing using CLSI methodology.11,16
Per-patient microbiological response (definitions available as Supplementary data) at the EOT and TOC visits was determined in the microbiologically evaluable (ME) population.
Blood samples for PK analysis were collected over an 8 h period on study Day 3 (exceptions were allowed for study sites without PK collection facilities). Most patients underwent sparse blood sample collection (four samples/patient); a subset underwent an intensive sample collection (12 samples/patient). Plasma drug concentrations were determined by a central laboratory (Covance Bioanalytical Laboratory, IN, USA) using validated methods. All samples were stored at −70°C until shipped to the central laboratory.
Outcomes
The primary endpoint was the proportion of patients clinically cured at the TOC visit in the co-primary CE and MITT populations (see Supplementary data for definitions). Secondary endpoints included the following: clinical response at TOC in the microbiological MITT (mMITT) and ME populations; clinical and per-pathogen microbiological response at TOC (ME population); clinical relapse and reinfection or recurrence at the LFU visit; the PK profile of ceftaroline fosamil 600 mg administered as a 2 h iv infusion every 8 h; and safety.
MRSA expansion period
An MRSA-focused expansion period was initiated following completion of the main trial (further details are available as Supplementary data).
Statistical analysis
The sample size estimation assumed a clinical cure rate at TOC of 80% in both treatment groups in the MITT population, a non-inferiority margin of 10% and power of 90%. This gives a total sample size of 765 patients (510 ceftaroline fosamil; 255 vancomycin plus aztreonam), which also gives >90% power for the CE population assuming 85% clinically cured in each group and a 20% non-evaluable rate. Approximately one-third of the way through the trial, a blinded review of evaluability and overall cure rates (both treatments combined) was undertaken to ensure the protocol assumptions held and the sample size was appropriate.
A two-sided 95% CI for the observed difference in the proportion of patients clinically cured at the TOC visit was computed using Miettinen and Nurminen's unstratified method.17 Non-inferiority of ceftaroline fosamil was defined as a lower limit of the 95% CI (corresponding to a 97.5% one-sided lower bound) greater than −10% for both the MITT and the CE population. All statistical analyses were conducted using SAS version 8.02 (SAS Institute, Cary, NC, USA). ClinicalTrials.gov registration numbers: NCT01499277 (main study) and NCT02202135 (expansion period).
Results
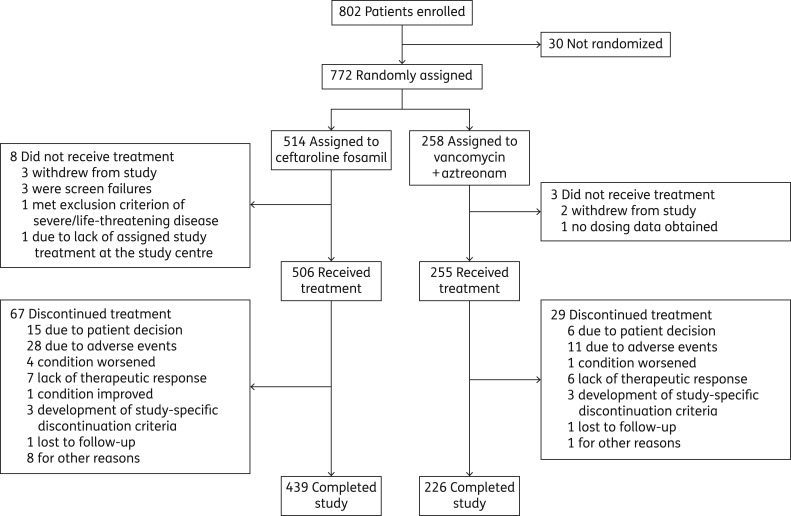
In total, 802 patients were enrolled, 772 were randomized and 761 received study treatment (MITT and safety population: ceftaroline fosamil, n = 506; vancomycin plus aztreonam, n = 255) (Figure 1; see Table S1 for details of study sites and total numbers of patients recruited by country). The CE population comprised 606 patients (ceftaroline fosamil, n = 395; vancomycin plus aztreonam, n = 211).
Figure 1.
Trial profile.
Demographic and baseline patient characteristics were well balanced across groups (Table 1). Cellulitis (57.3%) and major cutaneous abscess (21.3%) were the most common cSSTIs; most infections (94.1%) were community acquired.
Table 1.
Demographic and baseline characteristics of enrolled patients (MITT population)
| Parameter | Ceftaroline fosamil (n = 506) | Vancomycin + aztreonam (n = 255) |
|---|---|---|
| Age, mean (SD), years | 52.6 (16.5) | 53.6 (16.3) |
| Age group, n (%) | ||
| <65 years | 387 (76.5) | 183 (71.8) |
| ≥65 years | 119 (23.5) | 72 (28.2) |
| Sex, n (%) | ||
| Female | 196 (38.7) | 107 (42.0) |
| Male | 310 (61.3) | 148 (58.0) |
| BMI, mean (SD), kg/m2 | 27.6 (6.2) | 27.5 (5.9) |
| Race, n (%) | ||
| white | 341 (67.4) | 160 (62.7) |
| black or African American | 13 (2.6) | 13 (5.1) |
| Asian | 126 (24.9) | 64 (25.1) |
| American Indian or Alaska Native | 1 (0.2) | 0 |
| other | 25 (4.9) | 18 (7.1) |
| Type of infection, n (%) | ||
| cellulitis | 300 (59.3) | 136 (53.3) |
| traumatic or surgical wound infection | 63 (12.5) | 41 (16.1) |
| major cutaneous abscess | 103 (20.4) | 59 (23.1) |
| burn infection | 38 (7.5) | 18 (7.1) |
| other | 2 (0.4) | 1 (0.4) |
| Median lesion size, cm2 | 400.0 | 400.0 |
| Comorbid conditions, n (%) | ||
| DM | 84 (16.6) | 38 (14.9) |
| PVD | 27 (5.3) | 11 (4.3) |
| DM and PVD | 7 (1.4) | 3 (1.2) |
| HIV infection | 6 (1.2) | 2 (0.8) |
| renal impairment | 15 (3.0) | 5 (2.0) |
| cirrhosis | 1 (0.2) | 2 (0.8) |
| malnutritiona | 5 (1.0) | 5 (2.0) |
| use of immunosuppressive agents | 6 (1.2) | 4 (1.6) |
| malignancy other than non-melanoma skin cancers | 12 (2.4) | 5 (2.0) |
| Source of cSSTI infection, n (%) | ||
| community acquired | 479 (94.7) | 237 (92.9) |
| nosocomial | 12 (2.4) | 7 (2.7) |
| healthcare acquired | 14 (2.8) | 11 (4.3) |
| missing | 1 (0.2) | 0 |
| SIRS, n (%) | 199 (39.3) | 105 (41.2) |
| Fever (>38°C), n (%) | 211 (41.7) | 118 (46.3) |
| Heart rate >90 beats/min, n (%) | 178 (35.2) | 87 (34.1) |
| Respiratory rate >20 breaths/min, n (%) | 119 (23.5) | 57 (22.4) |
| Elevated WBCs (>12 000 cells/mm3), n (%) | 155 (30.6) | 71 (27.8) |
| Low WBCs (<4000 cells/mm3), n (%) | 10 (2.0) | 6 (2.4) |
| Presence of bacteraemia, n (%) | 18 (3.6) | 16 (6.3) |
| SIRS or bacteraemia, n (%) | 208 (41.1) | 113 (44.3) |
| CRP >50 mg/L, n (%) | 317 (62.6) | 148 (58.0) |
| At least one systemic sign at baselineb | 312 (61.7) | 166 (65.1) |
| Prior systemic antibiotics within 4 weeks of first dose of study drug | 240 (47.4) | 116 (45.5) |
DM, diabetes mellitus; PVD, peripheral vascular disease.
aMalnutrition was defined as baseline albumin <0.25 g/L in the absence of liver disease or baseline BMI <17 kg/m2.
bSystemic signs at baseline: fever >38°C, hypothermia <36°C, elevated WBCs >12 000 cells/mm3 or bands >10% at baseline.
Baseline pathogens were comparable between treatment groups, and were typical of cSSTI. The most frequently identified pathogens (mMITT population) are shown in Table S2. In the mMITT population, 223/384 (58.1%) patients had monomicrobial infections caused by Gram-positive pathogens. The most commonly isolated pathogen (primary infection site or blood) was Staphylococcus aureus [221/384 (57.6%); 168 MSSA and 54 MRSA (both MSSA and MRSA were isolated in one patient in the vancomycin plus aztreonam group)]. Enterobacteriaceae were isolated in 83/384 (21.6%) patients, including seven ESBL-positive isolates.
In vitro activities of ceftaroline, vancomycin and aztreonam against key baseline pathogens (mMITT population) are shown in Table 2; >95% were susceptible to ceftaroline according to CLSI breakpoints for ceftaroline fosamil 600 mg every 12 h11 (including MSSA and MRSA, for which all MIC values were ≤1 mg/L).
Table 2.
In vitro activity (MIC in mg/L) of study drugs against key baseline pathogens isolated from primary infection site or blood (mMITT population)
| Ceftaroline |
Vancomycin |
||||||
|---|---|---|---|---|---|---|---|
| Number of isolatesa | MIC range | MIC50b | MIC90b | MIC range | MIC50b | MIC90b | |
| Gram-positive pathogens | |||||||
| Staphylococcus aureus (total) | 217 | 0.06–1 | 0.25 | 0.5 | ≤0.25–1 | 0.5 | 1 |
| MSSA | 164 | 0.06–0.5 | 0.25 | 0.25 | ≤0.25–1 | 0.5 | 1 |
| MRSA | 54 | 0.25–1 | 0.5 | 0.5 | 0.5–1 | 0.5 | 1 |
| Streptococcus pyogenes | 25 | ≤0.008 | ≤0.008 | ≤0.008 | ≤0.25 | ≤0.25 | ≤0.25 |
| Streptococcus anginosus group | 24 | ≤0.008–0.03 | 0.03 | 0.03 | 0.5–1 | 0.5 | 1 |
| Enterococcus faecalis | 19 | 0.5–64 | 1 | 8 | 0.5–1 | 1 | 2 |
| Streptococcus agalactiae | 16 | ≤0.008–0.15 | 0.15 | 0.15 | ≤0.25–0.5 | 0.5 | 0.5 |
| Streptococcus dysgalactiae | 12 | ≤0.008–0.06 | ≤0.008 | 0.15 | ≤0.25–1 | ≤0.25 | 1 |
| Ceftaroline |
Aztreonam |
||||||
| Number of isolatesa | MIC range | MIC50b | MIC90b | MIC range | MIC50b | MIC90b | |
| Gram-negative pathogens | |||||||
| Escherichia coli | 30 | 0.015 to >32 | 0.12 | 16 | ≤0.03 to >32 | 0.12 | 4 |
| ESBL negative | 26 | ≤0.03–8 | 0.06 | 1 | ≤0.03–4 | 0.06 | 0.12 |
| Klebsiella pneumoniae | 12 | 0.06 to >32 | 0.25 | >32 | ≤0.03 to >32 | 0.06 | >32 |
| ESBL negative | 8 | 0.06–2 | NA | NA | ≤0.03–0.25 | NA | NA |
| Proteus mirabilis | 11 | 0.03 to >32 | 0.06 | 0.12 | ≤0.03–0.12 | ≤0.03 | 0.12 |
| Pseudomonas aeruginosa | 9 | 1 to >32 | NA | >32 | NA | 8 | NA |
| Klebsiella oxytoca | 8 | 0.03–0.25 | NA | NA | ≤0.03–0.25 | NA | NA |
| Morganella morganii | 7 | 0.03–0.12 | NA | NA | ≤0.03–0.12 | NA | NA |
NA, not assessed because there were <10 isolates.
aWhere there were multiple isolates for a single patient, the isolate with the highest MIC value was used. Isolates of both MSSA and MRSA from a single patient were counted only once in the total for S. aureus.
bMIC50 and MIC90 calculated for isolates where n ≥ 10.
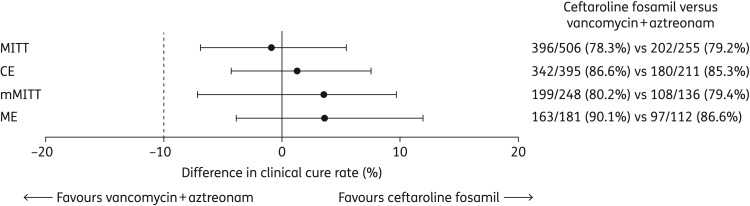
The proportion of patients clinically cured at the TOC visit is shown in Figure 2. Non-inferiority of ceftaroline fosamil 600 mg every 8 h versus vancomycin plus aztreonam was demonstrated, with between-group differences (95% CI) for the MITT and CE populations of −0.95% (–6.90, 5.41) and 1.27% (–4.32, 7.48), respectively.
Figure 2.
Clinical cure rates at the TOC visit and 95% CIs for the between-group differences. Dashed line at −10% reflects non-inferiority margin; MITT and CE are the primary analysis populations.
Clinical cure rates by infection type and in patients with markers of severe disease, including systemic inflammatory response syndrome (SIRS), bacteraemia and elevated C-reactive protein (CRP), were generally comparable between treatment arms, and there were no meaningful shifts in clinical outcome by infection type or disease severity parameters compared with the overall MITT population (Table 3). Similar results were observed in the CE population (data not shown). Clinical response rates in the mMITT and ME populations were consistent with the CE/MITT co-primary analysis populations (Figure 2).
Table 3.
Clinical cure rates by cSSTI infection type and disease severity (MITT population)
| Ceftaroline fosamil n/N (%) | Vancomycin+aztreonam n/N (%) | Difference, % (95% CI) | |
|---|---|---|---|
| cSSTI infection type | |||
| cellulitis | 230/300 (76.7) | 99/136 (72.8) | 3.87 (–4.66, 13.09) |
| traumatic or surgical wound infection | 49/63 (77.8) | 36/41 (87.8) | –10.03 (–24.14, 5.84) |
| major cutaneous abscess | 83/103 (80.6) | 49/59 (83.1) | –2.47 (–14.18, 10.85) |
| burn infection | 33/38 (86.8) | 18/18 (100.0) | –13.16 (–27.48, 5.48) |
| Disease severity | |||
| SIRS | 168/199 (84.4) | 83/105 (79.0) | 5.37 (–3.45, 15.23) |
| bacteraemia | 14/18 (77.8) | 10/16 (62.5) | 15.28 (–15.79, 44.58) |
| SIRS or bacteraemia | 174/208 (83.7) | 88/113 (77.9) | 5.78 (–2.98, 15.43) |
| fever (temperature >38°C) | 179/211 (84.8) | 104/118 (88.1) | –3.30 (–10.60, 4.92) |
| ≥2 severe local signsa | 61/84 (72.6) | 33/45 (73.3) | –0.71 (–15.95, 16.16) |
| ≤1 systemic signb | 255/312 (81.7) | 137/166 (82.5) | –0.80 (–7.71, 6.79) |
| elevated WBCs (>12 000 cells/mm3) | 122/155 (78.7) | 53/71 (74.6) | 4.06 (–7.26, 16.75) |
| baseline CRP (mg/L) | |||
| ≤50 | 149/178 (83.7) | 83/100 (83.0) | 0.71 (–8.04, 10.52) |
| >50 to ≤150 | 136/178 (76.4) | 67/80 (83.8) | –7.35 (–16.93, 3.77) |
| >150 | 105/139 (75.5) | 49/68 (72.1) | 3.48 (–8.75, 16.89) |
| ≥2 severe systemic signsb or fever (>38°C) or elevated WBCs (>10 000 cells/mm3) | 343/432 (79.4) | 174/219 (79.5) | –0.05 (–6.40, 6.79) |
aErythema, swelling, tenderness or warmth assessed by the investigator as ‘severe’.
bSystemic signs were fever >38°C, hypothermia <36°C, elevated WBC count >12 000 cells/mm3 or bands >10%.
Clinical cure rates were generally similar across geographic regions; however, they were lower in patients from Asian countries [MITT, 66.9% and 73.0% (95% CI, −19.14, 8.29); CE, 76.3% and 75.5% (95% CI, −12.83, 15.98) for ceftaroline fosamil and vancomycin plus aztreonam, respectively] than in patients from non-Asian regions [MITT, 81.9% and 81.3% (95% CI, −5.78, 7.76); CE, 89.9% and 88.6% (95% CI, −4.37, 7.93)]. This may partly reflect the higher incidence of clinical failure resulting from discontinuation of study therapy due to an AE in patients from Asian countries [ceftaroline fosamil, 11/124 (8.9%); vancomycin plus aztreonam, 4/63 (6.3%)] compared with non-Asian countries [4/382 (1.0%) and 3/192 (1.6%), respectively].
Of all randomized patients, 2/772 (0.3%) were lost to follow-up. Among patients who were clinically cured at TOC (CE population), relapse at LFU occurred in 3/342 (0.9%) ceftaroline fosamil and 3/180 (1.7%) vancomycin plus aztreonam patients. No new infections, reinfections or recurrences of infection occurred.
Microbiological responses were predominantly derived from clinical responses; therefore, clinical and microbiological response rates at TOC by baseline pathogen were similar. Clinical and microbiological response rates were similar across treatment groups for patients with monomicrobial and polymicrobial infections, and for those with key baseline pathogens, including MRSA (Table 4).
Table 4.
Favourable clinical and microbiological response at the TOC visit by key baseline pathogen from site of skin infection and infection composition (ME population)
| Favourable clinical response, n/N (%)a |
Favourable microbiological response, n/N (%)a |
|||
|---|---|---|---|---|
| ceftaroline fosamil (n = 181) | vancomycin + aztreonam (n = 112) | ceftaroline fosamil (n = 181) | vancomycin + aztreonam (n = 112) | |
| Gram-positive pathogens | ||||
| Staphylococcus aureus | ||||
| MSSA | 88/94 (93.6) | 49/57 (86.0) | 91/94 (96.8) | 49/57 (86.0) |
| MRSA | 21/25 (84.0) | 12/15 (80.0) | 22/25 (88.0) | 12/15 (80.0) |
| Streptococcus pyogenes | 14/15 (93.3) | 7/7 (100.0) | 14/15 (93.3) | 7/7 (100.0) |
| Streptococcus agalactiae | 5/6 (83.3) | 7/9 (77.8) | 6/6 (100.0) | 9/9 (100.0) |
| Streptococcus dysgalactiae | 9/9 (100.0) | 0 | 9/9 (100.0) | 0 |
| Enterococcus faecalis | 4/6 (66.7) | 4/5 (80.0) | 5/6 (83.3) | 4/5 (80.0) |
| Gram-negative pathogens | ||||
| Escherichia coli | 11/12 (91.7) | 9/10 (90.0) | 12/12 (100.0) | 9/10 (90.0) |
| Klebsiella pneumoniae | 5/7 (71.4) | 3/4 (75.0) | 6/7 (85.7) | 3/4 (75.0) |
| Klebsiella oxytoca | 4/4 (100.0) | 1/1 (100.0) | 4/4 (100.0) | 1/1 (100.0) |
| Proteus mirabilis | 6/7 (85.7) | 2/2 (100.0) | 6/7 (85.7) | 2/2 (100.0) |
| Morganella morganii | 4/4 (100.0) | 2/2 (100.0) | 4/4 (100.0) | 2/2 (100.0) |
| Infection composition | ||||
| monomicrobial | 113/125 (90.4) | 69/80 (86.3) | 116/125 (92.8) | 69/80 (86.3) |
| polymicrobial | 50/56 (89.3) | 28/32 (87.5) | 51/56 (91.1) | 29/32 (90.6) |
aPercentages are based on total number in the treatment group with the baseline pathogen.
Clinical cure and favourable microbiological response rates by ceftaroline MIC for key baseline pathogens are summarized for the ceftaroline fosamil group in Tables S3 and S4.
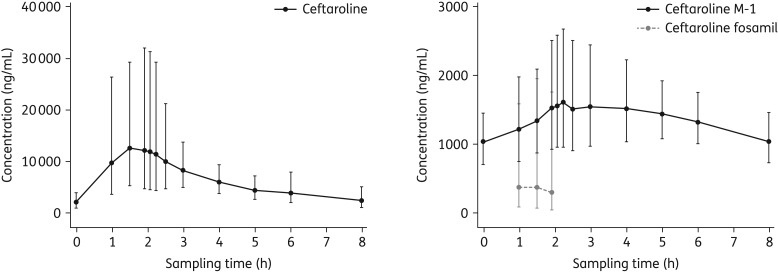
Of the 514 ceftaroline fosamil-treated patients, 396 (77.0%) were included in the PK population: 379 and 17 underwent sparse and intensive sampling, respectively. Plasma concentration–time profiles of ceftaroline, ceftaroline fosamil and the inactive metabolite ceftaroline M-1 (intensive sampling group) are shown in Figure 3. Geometric mean (% coefficient of variation; CV) areas under the plasma concentration–time curve from zero to the last measurable concentration (AUC0–t) for ceftaroline, ceftaroline fosamil and ceftaroline M-1 were 58.2 (52.3), 1.7 (270.8) and 11.0 mg/L (27.8), respectively. Respective Cmax values (%CV) were 19.1 (38.2), 1.1 (154.5) and 1.8 mg/L (36.6).
Figure 3.
Geometric mean ceftaroline, ceftaroline fosamil and ceftaroline M-1 plasma concentration–time profiles following a 2 h iv infusion of 600 mg of ceftaroline fosamil on Day 3 for patients who underwent intensive PK sampling (PK population). Vertical bars represent geometric SD.
The study treatments were generally well tolerated, and AEs were consistent with their known safety profiles. The incidence of AEs (safety population) was similar in each group: 232/506 (45.8%) ceftaroline fosamil and 116/255 (45.5%) vancomycin plus aztreonam recipients experienced one or more AEs. AEs reported by ≥1% of patients in either group are shown in Table 5. AEs were predominantly (>96%) of mild or moderate intensity.
Table 5.
Adverse events with a total frequency ≥1% in either treatment group categorized by MedDRA preferred term (safety population)
| Patients, n (%) |
||
|---|---|---|
| Preferred term | Ceftaroline fosamil (n = 506) | Vancomycin + aztreonam (n = 255) |
| Patients with an adverse event with a total frequency ≥1% | 142 (28.1) | 87 (34.1) |
| Nausea | 20 (4.0) | 11 (4.3) |
| Headache | 17 (3.4) | 12 (4.7) |
| Hypokalaemia | 15 (3.0) | 8 (3.1) |
| Vomiting | 13 (2.6) | 5 (2.0) |
| Diarrhoea | 12 (2.4) | 5 (2 0) |
| Anaemia | 10 (2.0) | 9 (3.5) |
| Dizziness | 10 (2.0) | 1 (0.4) |
| Rash | 10 (2.0) | 6 (2.4) |
| Constipation | 9 (1.8) | 8 (3.1) |
| Pyrexia | 9 (1.8) | 5 (2.0) |
| Drug eruption | 8 (1.6) | 0 |
| Infusion site phlebitis | 8 (1.6) | 5 (2.0) |
| Insomnia | 7 (1.4) | 4 (1.6) |
| Oedema peripheral | 7 (1.4) | 4 (1.6) |
| Rash generalized | 7 (1.4) | 4 (1.6) |
| Alanine aminotransferase increased | 6 (1.2) | 4 (1.6) |
| Hypertension | 6 (1.2) | 8 (3.1) |
| Rash pruritic | 6 (1.2) | 0 |
| Anxiety | 5 (1.0) | 3 (1.2) |
| Aspartate aminotransferase increased | 5 (1.0) | 5 (2.0) |
| Dyspnoea | 5 (1.0) | 3 (1.2) |
| Urticaria | 5 (1.0) | 3 (1.2) |
| Hepatic enzyme increased | 4 (0.8) | 7 (2.7) |
| Pruritus generalized | 4 (0.8) | 5 (2.0) |
| Asthenia | 2 (0.4) | 3 (1.2) |
| Diabetes mellitus | 2 (0.4) | 3 (1.2) |
| Oropharyngeal pain | 2 (0.4) | 4 (1.6) |
| Abdominal distension | 1 (0.2) | 4 (1.6) |
| Iron deficiency anaemia | 1 (0.2) | 3 (1.2) |
| Hypomagnesaemia | 0 | 3 (1.2) |
| Pruritus | 0 | 4 (1.6) |
| Type 2 diabetes mellitus | 0 | 3 (1.2) |
AEs were considered treatment-related (TRAEs) in 81/506 (16.0%) and 42/255 (16.5%) ceftaroline fosamil and vancomycin plus aztreonam recipients, respectively. TRAEs reported in ≥1% of patients were nausea, vomiting, drug eruption, diarrhoea (including one case of Clostridium difficile colitis in one patient in the ceftaroline fosamil group), rash, headache and infusion site phlebitis in the ceftaroline fosamil group, and infusion site phlebitis, headache, nausea, increased AST, increased hepatic enzyme, increased ALT, constipation and pruritus generalized in the vancomycin plus aztreonam group. TRAEs that differed in frequency by ≥1% between the ceftaroline fosamil and vancomycin plus aztreonam groups were increased AST (0.6% and 1.6%, respectively), increased hepatic enzyme (0.6% and 1.6%), infusion site phlebitis (1.0% and 2.0%), drug eruption (1.4% and 0%), rash (1.2% and 0%) and constipation (0% and 1.2%). The frequency of severe TRAEs was similar across groups [ceftaroline fosamil, 5/506 (1.0%); vancomycin plus aztreonam, 3/255 (1.2%)] and comprised drug hypersensitivity, hypokalaemia, drug eruption, urticaria and increased hepatic enzyme (ceftaroline fosamil), and urticaria, toxic nephropathy and pyrexia (vancomycin plus aztreonam).
Overall, 96/761 (12.6%) patients prematurely discontinued treatment [ceftaroline fosamil, 67 (13.2%); vancomycin plus aztreonam, 29 (11.4%)], of whom 32/506 (6.3%) and 11/255 (4.3%) patients, respectively, had one or more AEs leading to discontinuation; these were considered treatment-related in 22 and 7 patients, respectively. AEs leading to discontinuation in >1% of patients in either group were in the system organ classes of disorders of skin and subcutaneous tissue [19/506 (3.8%) versus 3/255 (1.2%)]; infections and infestations [5/506 (1.0%) versus 1/255 (0.4%)]; and investigations [2/506 (0.4%) versus 3/255 (1.2%)].
The incidence of AEs representing rash events (including the preferred terms dermatitis, dermatitis allergic, drug eruption, erythema, generalized erythema, rash, rash erythematous, rash generalized, rash maculopapular, rash papular and rash pruritic) were grouped together as an AE of special interest. Rash events were reported more frequently for ceftaroline fosamil [44/506 (8.7%)] than for vancomycin plus aztreonam [5.1% (13/255)]; >92% were of mild or moderate intensity, and treatment-related rash events occurred in 30/506 (5.9%) and 4/255 (1.6%) patients, respectively. All rash events were reversible and none was associated with Stevens–Johnson syndrome or toxic epidermal necrolysis. Rash events associated with ceftaroline fosamil were typically described as maculopapular and were distributed over the trunk and extremities; this is consistent with the pattern of cephalosporin-induced rash.18
The imbalance in the frequency of rash was driven by the frequencies of these events in patients from Asian sites: AEs of rash occurred in 23/124 (18.5%) ceftaroline fosamil-treated patients at Asian sites and 5/63 (7.9%) of vancomycin plus aztreonam recipients. When Asian patients were excluded, the frequency of rash was similar between groups [21/382 (5.5%) and 8/192 (4.2%), respectively]. In the ceftaroline fosamil group, there was a bimodal distribution in the time to onset of rash, with peaks at Days 3–4 (early-onset events) and Days 8–9 (late-onset events). The median duration of treatment in Asian patients in both treatment groups was longer than in non-Asian patients (8.6 versus 6.8 days for ceftaroline fosamil; 8.8 versus 6.8 days for vancomycin plus aztreonam). Among those who received treatment for ≥8 days, 17/85 (20.0%) of Asian patients and 11/151 (7.3%) of non-Asian patients experienced late-onset rash. The incidence of rash occurring during the first 7 days was much lower and in line with that seen previously.2,3,19 Positive Coombs seroconversion occurred in 146/452 (32.3%) patients in the ceftaroline fosamil group at TOC; none of these patients showed evidence of drug-induced haemolytic anaemia. A summary of AEs in the MRSA-expansion period is presented in Table S5.
Discussion
These results provide valuable information regarding the potential clinical utility of ceftaroline fosamil 600 mg every 8 h. The primary efficacy endpoint was met, demonstrating the non-inferiority of ceftaroline fosamil 600 mg every 8 h versus vancomycin plus aztreonam in patients with cSSTI with evidence of systemic inflammatory response or underlying comorbidities associated with impaired immune response in the co-primary MITT and CE populations.
Moreover, clinical cure rates at TOC among patients with evidence of more severe disease at baseline (including SIRS, bacteraemia and elevated CRP) were generally comparable between treatment groups, and were similar to the overall cure rate. Clinical and microbiological cure rates (ME population) for key cSSTI pathogens were generally comparable across treatment groups. In line with previous surveillance studies20,21 and clinical trials,2,3 the most commonly isolated pathogen was S. aureus. Consistent with the previous trials,2,3 ceftaroline fosamil was effective against infections caused by MRSA. However, relatively few MRSA were isolated (13.8% in the mMITT population). This may reflect the predominance of community-acquired infections (94.7%; hospital-acquired infections would be unlikely to progress untreated to such a stage that they met the stringent severity inclusion criteria for this study) and the small proportion (15.5%) of patients from the USA (∼60% of MRSA isolates were from the USA).
Patients in COVERS had a larger area of cutaneous involvement compared with CANVAS (median lesion size 400 versus 151 cm2), and a greater proportion had evidence of systemic inflammation (∼40% versus 23% had SIRS). Despite the increase in dose from 600 mg every 12 h in CANVAS 1 and 2 to 600 mg every 8 h in COVERS, overall clinical cure rates were somewhat lower in COVERS compared with CANVAS. Nevertheless, clinical outcomes among ceftaroline fosamil recipients with evidence of more severe disease were generally comparable for COVERS and CANVAS; clinical cure rates in patients with one or more systemic signs, fever, elevated white blood cells (WBCs) or SIRS differed by <5% across the trials.22 Moreover, per-pathogen clinical response rates were also generally comparable across the three trials, and MICs of >95% for isolated pathogens were within respective CLSI susceptibility breakpoints for the ceftaroline fosamil dose of 600 mg every 12 h.23 Considered together, these data suggest that ceftaroline fosamil 600 mg every 12 h is a robust treatment for the majority of patients with cSSTI, including those with evidence of SIRS or underlying comorbidities.
The PK profile of ceftaroline fosamil every 8 h was in line with expectations. Geometric mean ceftaroline AUC and Cmax values were within ∼10%–20% of those reported in Chinese and Western healthy volunteers assessed after 8 days of ceftaroline fosamil 600 mg every 8 h in 2 h infusions.19 A population PK model that incorporated the patient PK data from COVERS was used to analyse ceftaroline exposures: consistent with the clinical efficacy findings, the PK profile of ceftaroline was unaffected by the presence of one or more systemic signs of inflammation, fever, elevated WBCs or SIRS.24 In Monte Carlo simulations, ceftaroline fosamil 600 mg every 12 h provided exposures associated with the plateau of dose–response for efficacy against S. aureus (including MRSA) with MICs up to 2 mg/L,25 and ceftaroline fosamil 600 mg every 8 h achieved >95% target attainment against S. aureus with MICs of 4 mg/L.26
The safety profile of ceftaroline fosamil 600 mg every 8 h was similar to those in previous trials of 600 mg every 12 h,7,19,27,28 with no new safety signals identified. Rash was a common cause of discontinuation, and occurred more frequently among Asian patients than non-Asian patients treated with ceftaroline fosamil. Median duration of ceftaroline fosamil treatment was longer in Asian patients (8.6 days) compared with non-Asian patients (6.8 days). However, even considering only those who received treatment for ≥8 days, a greater proportion of Asian patients (20.0%) relative to non-Asian patients (7.3%) had late-onset rash. Hypersensitivity reactions are a well-documented adverse effect associated with β-lactams, and regional variations in usage may influence sensitization patterns.29,30 Rash events were the most frequent AEs reported in a Phase I trial in Chinese healthy volunteers, and occurred at a similar frequency in patients receiving ceftaroline fosamil 600 mg every 12 or 8 h.19 Interestingly, the majority [9/16 (56.3%)] of these AEs were late in onset (i.e. on or after study Day 7). In a similar Phase I trial in Western healthy volunteers, a typical cephalosporin maculopapular rash also occurred; however, the frequency and intensity of rash AEs were higher in Chinese compared with Western volunteers.19 It is possible that physiological differences increase the propensity of Asian subjects to developing cutaneous reactions to antibiotics.31
The frequency of a positive Coombs seroconversion test was high (32.3% at TOC) in the ceftaroline fosamil group compared with the CANVAS trials; this may reflect the dose and treatment duration. Importantly, across the cephalosporin class, positive Coombs seroconversion has low predictive value for drug-induced haemolytic anaemia.32 Moreover, no associated cases of drug-induced haemolytic anaemia were reported.
A key limitation was the absence of MRSA with ceftaroline MICs of ≥2 mg/L (with the exception of one patient in the expansion period). This appears surprising given that MRSA MIC90 values of 2 mg/L have been reported in some regions (e.g. Asia, Latin America and some European countries) where the study was conducted.8–10 The stringent study entry criteria (which followed regulatory guidance),33 specifically the requirement for a large area of infection involvement (minimum 75 cm2) and limiting prior antibiotic exposure, may have biased recruitment towards community-acquired infections, which typically involve MRSA with ceftaroline MICs ≤1 mg/L.
In conclusion, ceftaroline fosamil 600 mg every 8 h was effective and generally well tolerated in the treatment of patients with cSSTI characterized by large areas of body surface involvement and evidence of systemic inflammation and/or underlying comorbidities, and no new safety concerns were identified. Importantly, disease severity did not appear to affect clinical cure rates. Further evaluation is required to establish whether ceftaroline fosamil every 8 h might extend coverage to S. aureus with higher ceftaroline MICs.
Funding
This study was funded by AstraZeneca. Medical writing support was provided by Elizabeth Bolton (née Evans) and Mark Waterlow of Prime Medica Ltd, Knutsford, Cheshire, UK, funded by AstraZeneca. The design and conduct of the study, as well as analysis of the study data and opinions, conclusions and interpretation of the data, are the responsibility of the authors. Ceftaroline fosamil is being developed by AstraZeneca and Actavis (an affiliate of Allergan Inc).
Transparency declarations
The authors are employees of AstraZeneca (D. W. and J. P. I.) or former employees of AstraZeneca (J. G.) or have received institutional/research grant funding for the conduct of the study (M. D., Y. Z.).
Author contributions
M. D. was the principal investigator for this study, and was involved in study design and data interpretation. Y. Z. was the Chinese National Principal Investigator for this study, and contributed to the study design. D. W., J. P. I. and J. G. contributed to the study design and data interpretation, and D. W. did the data analyses. J. P. I. contributed to the analysis of microbiological data. All authors contributed to each stage of the development of the report and reviewed and approved the final version for submission.
Supplementary data
Supplementary Methods and Tables S1 to S5 are available as Supplementary data at JAC Online (http://jac.oxfordjournals.org.uk/).
Acknowledgements
The authors would like to thank all of the patients who participated in the trial, and the investigators and study site staff at each of the participating sites for their contributions. A preliminary report of these data was presented at the 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), 25–28 April 2015, Copenhagen, Denmark (Abstract O193).
References
- 1.Edelsberg J, Taneja C, Zervos M et al. . Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 2009; 15: 1516–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corey GR, Wilcox MH, Talbot GH et al. . CANVAS 1: the first Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 2010; 65 Suppl 4: iv41–iv51. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox MH, Corey GR, Talbot GH et al. . CANVAS 2: the second Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 2010; 65 Suppl 4: iv53–iv65. [DOI] [PubMed] [Google Scholar]
- 4.Eckmann C, Dryden M. Treatment of complicated skin and soft-tissue infections caused by resistant bacteria: value of linezolid, tigecycline, daptomycin and vancomycin. Eur J Med Res 2010; 15: 554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AstraZeneca AB. Zinforo 600 mg Powder for Concentrate for Solution for Infusion. Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002252/WC500132586.pdf.
- 6.Corey GR, Wilcox M, Talbot GH et al. . Integrated analysis of CANVAS 1 and 2: phase 3, multicenter, randomized, double-blind studies to evaluate the safety and efficacy of ceftaroline versus vancomycin plus aztreonam in complicated skin and skin-structure infection. Clin Infect Dis 2010; 51: 641–50. [DOI] [PubMed] [Google Scholar]
- 7.Corrado ML. Integrated safety summary of CANVAS 1 and 2 trials: Phase III, randomized, double-blind studies evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 2010; 65 Suppl 4: iv67–iv71. [DOI] [PubMed] [Google Scholar]
- 8.Flamm RK, Sader HS, Jones RN. Ceftaroline activity tested against contemporary Latin American bacterial pathogens (2011). Braz J Infect Dis 2014; 18: 187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flamm RK, Jones RN, Sader HS. In vitro activity of ceftaroline tested against isolates from the Asia-Pacific region and South Africa (2011). J Global Antimicrob Resist 2014; 2: 183–9. [DOI] [PubMed] [Google Scholar]
- 10.Castanheira M, Jones RN, Sader HS. Activity of ceftaroline and comparator agents tested against contemporary Gram-positive and -negative (2011) isolates collected in Europe, Turkey, and Israel. J Chemother 2014; 26: 202–10. [DOI] [PubMed] [Google Scholar]
- 11.Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Third Informational Supplement M100-S23. CLSI, Wayne, PA, USA, 2013. [Google Scholar]
- 12.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 5.0, 2015 www.eucast.org/clinical_breakpoints/.
- 13.Pinder M, Bellomo R, Lipman J. Pharmacological principles of antibiotic prescription in the critically ill. Anaesth Intensive Care 2002; 30: 134–44. [DOI] [PubMed] [Google Scholar]
- 14.Murray PR, Baron EJ, Jorgensen JH et al. . Manual of Clinical Microbiology. Washington, DC: ASM Press, 2007. [Google Scholar]
- 15.Levine NS, Lindberg RB, Mason AD Jr et al. . The quantitative swab culture and smear: a quick, simple method for determining the number of viable aerobic bacteria on open wounds. J Trauma 1976; 16: 89–94. [PubMed] [Google Scholar]
- 16.Clinical Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically—Ninth Edition: Approved Standard M07-A9. CLSI, Wayne, PA, USA, 2012. [Google Scholar]
- 17.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985; 4: 213–26. [DOI] [PubMed] [Google Scholar]
- 18.Swanbeck G, Dahlberg E. Cutaneous drug reactions. An attempt to quantitative estimation. Arch Dermatol Res 1992; 284: 215–8. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Sunzel M, Xu P et al. . Evaluation of the pharmacokinetics and safety of single and multiple ceftaroline fosamil infusions in healthy Chinese and Western subjects. Int J Clin Pharmacol Ther 2015; 53: 681–91. [DOI] [PubMed] [Google Scholar]
- 20.Diekema DJ, Pfaller MA, Schmitz FJ et al. . Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis 2001; 32 Suppl 2: S114–S32. [DOI] [PubMed] [Google Scholar]
- 21.Moet GJ, Jones RN, Biedenbach DJ et al. . Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998-2004). Diagn Microbiol Infect Dis 2007; 57: 7–13. [DOI] [PubMed] [Google Scholar]
- 22.Corey R, Wilcox M, Gonzalez J et al. . Ceftaroline fosamil (CPT-F) in patients with acute bacterial skin and skin structure infections (ABSSSI) with systemic inflammatory signs: results across 3 pivotal studies using q8h or q12h. In: Abstracts of the Fifty-fifth Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, USA, 2015. p. L-839. American Society for Microbiology. [Google Scholar]
- 23.Reiszner E, Ambler J, Iaconis J. Ceftaroline in the treatment of complicated skin and soft tissue infections: in vitro susceptibility of baseline pathogens isolated in a Phase III randomised clinical trial. In: Abstracts of the Twenty-fifth European Congress of Clinical Microbiology and Infection. Copenhagen, Denmark, 2015. p. EV0128. European Society of Clinical Microbiology and Infectious Diseases. [Google Scholar]
- 24.Zhou D, Dryden M, Gonzalez J et al. . Impact of disease severity on ceftaroline pharmacokinetics (PK) in patients with ABSSSI: Phase III COVERS trial. In: Abstracts of the Fifty-fifth Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, USA, 2015. p. A-966. American Society for Microbiology. [Google Scholar]
- 25.Iaconis J, Critchley I, Zhou D et al. . Ceftaroline fosamil 600 mg every 12h (every 12 h ) can provide adequate exposure against Staphylococcus aureus with ceftaroline MICs ≤2mg/L in ABSSSI. In: Abstracts of the Fifty-fifth Interscience Conference on Antimicrobial Agents and Chemotherapy. San Diego, CA, USA, 2015. p. A-456. American Society for Microbiology. [Google Scholar]
- 26.Das S, Li J, Gonzalez J et al. . Ceftaroline fosamil 600mg every 8h for the treatment of ABSSSI due to Staphylococcus aureus with ceftaroline MICs of 4 mg/L. In: Abstracts of the Fifty-fifth Interscience Conference on Antimicrobial Agents and Chemotherapy. San Diego, CA, USA, 2015. p. A-455. American Society for Microbiology. [Google Scholar]
- 27.Rank DR, Friedland HD, Laudano JB. Integrated safety summary of FOCUS 1 and FOCUS 2 trials: Phase III randomized, double-blind studies evaluating ceftaroline fosamil for the treatment of patients with community-acquired pneumonia. J Antimicrob Chemother 2011; 66 Suppl 3: iii53–iii9. [DOI] [PubMed] [Google Scholar]
- 28.Zhong NS, Sun T, Zhuo C et al. . Ceftaroline fosamil versus ceftriaxone for the treatment of Asian patients with community-acquired pneumonia: a randomised, controlled, double-blind, phase 3, non-inferiority with nested superiority trial. Lancet Infect Dis 2015; 15: 161–71. [DOI] [PubMed] [Google Scholar]
- 29.Dona I, Barrionuevo E, Blanca-Lopez N et al. . Trends in hypersensitivity drug reactions: more drugs, more response patterns, more heterogeneity. J Investig Allergol Clin Immunol 2014; 24: 143–53. [PubMed] [Google Scholar]
- 30.Rodriguez-Pena R, Antunez C, Martin E et al. . Allergic reactions to β-lactams. Expert Opin Drug Saf 2006; 5: 31–48. [DOI] [PubMed] [Google Scholar]
- 31.Thong BY, Tan TC. Epidemiology and risk factors for drug allergy. Br J Clin Pharmacol 2011; 71: 684–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andes DR, Craig WA. Cephalosporins. In: Mandell GL, ed. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Philadelphia: Elsevier Inc, 2005; 294–311. [Google Scholar]
- 33.Food and Drug Administration, Center for Drug Evaluation Research. Guidance for Industry. Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment. 2013. http://www.fda.gov/downloads/Drugs/.../Guidances/ucm071185.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.