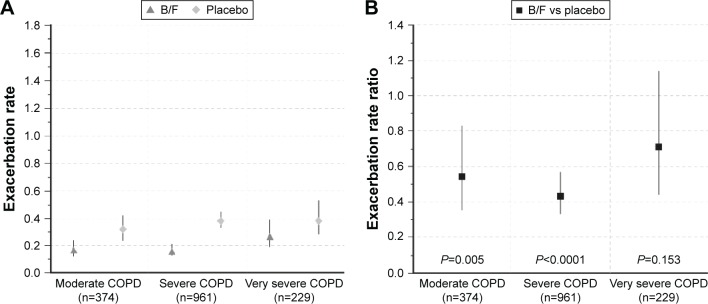
Figure 2.
(A) 3-month exacerbation rates in patients with moderate, severe, and very severe COPD receiving B/F or placebo; and (B) 3-month exacerbation rate ratios between B/F and placebo, by disease severity. Vertical lines represent 95% confidence intervals. Moderate COPD was defined as post-bronchodilator FEV1 80%–50% predicted, severe COPD as post-bronchodilator FEV1 50%–30% predicted, and very severe COPD as post-bronchodilator FEV1 <30% predicted, according to GOLD 2016 criteria (3-month data set; Calverley et al, Szafranski et al, and CLIMB studies).9,10,12
Abbreviations: B/F, budesonide/formoterol; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s.