Abstract
Objectives
To assess the association of adipocyte size with cellular lipolysis, and between cellular lipolysis and whole body lipid oxidation. Then, to assess the association between adipocyte size and cellular lipolysis with weight and fat mass gain.
Methods
Subjects had assessment of percent body fat (%fat) and adipose tissue biopsy for in vitro lipolysis (n=325), and a subset of subjects had measurement of whole body lipid oxidation (n=112). A subset of subjects (n=243) returned for repeat measurements of body weight and composition (mean follow-up 8.2 ± 5.5 years).
Results
In vitro lipolysis (r=0.47, p<0.0001) and adipocyte size (r=0.49, p<0.0001) were strongly associated with %fat. In vitro lipolysis (p=0.04) but not adipocyte size (p=0.44) was associated with whole body fat oxidation. Adipocyte size was not associated with rate of percent weight gain (p=0.20), but was negatively associated with rate of percent fat mass gain (p=0.01). In vitro lipolysis was negatively associated with rate of percent weight gain (p=0.02) and had a marginal negative association with rate of percent fat mass gain (p=0.08).
Conclusions
These results indicate inherent characteristics of adipocytes, including size and lipolytic activity, may be important determinants of whole body lipid oxidation and subsequent weight gain.
Keywords: Pima Indian, weight gain, lipid metabolism, adipocytes, lipolysis
Introduction
Positive energy balance results in greater triglyceride storage in adipose tissue and resultant accumulation of body fat. Increased fat causes adipocytes to increase in size and release more lipids1,2. Genetic polymorphisms at several loci have been associated with both adipocyte lipolysis and obesity, indicating a possible connection between adipocyte function and weight. However, direct association between basal adipocyte lipolysis and weight has not been studied in humans.
Adipocyte lipolysis may contribute to the rate of whole body lipid oxidation3. This may occur by the liberation of free fatty acids and glycerol, which may provide increased substrate for mitochondrial beta-oxidation. Evidence indicates a role for whole body lipid oxidation in long-term weight regulation. Elevated respiratory quotient (RQ), which indicates greater relative carbohydrate to fat oxidation, predicts weight gain in various populations4,5,6. Whole body lipid oxidation is negatively associated with weight gain in Native American men, but not women4. However, Pima Indians and Caucasians with high levels of adiposity have higher lipid oxidation rates than their lean counterparts7. Nevertheless, positive cross-sectional associations do not preclude these variables as negative determinants of future weight gain8. Thus if adipocyte lipolysis is associated with whole body lipid oxidation this would provide a mechanism for any association with weight change.
The purpose of this study was to first to confirm the association of adipocyte size with cellular lipolysis in Pima Indians, and to investigate the association between adipocyte size and cellular lipolysis with whole body lipid oxidation. We then investigated whether adipocyte size and in vitro cellular lipolysis were determinants of weight and in particular fat mass gain.
Methods
Subjects and Protocol
Volunteers (n = 325) participated in an inpatient study investigating risk factors for type 2 diabetes at the Obesity and Diabetes Clinical Research Section of the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) in Phoenix, AZ. Volunteers were told about the purpose of the study and written informed consent was obtained before participation. The Institutional Review Board of the NIDDK approved all protocols.
Volunteers were admitted to the clinical research unit and fed a weight maintaining diet. On day 2 of admission, participants underwent measurement of body composition using under water weighing9. On day 4 a 75-gram oral glucose tolerance test was administered, and subjects with diabetes mellitus were excluded from the study. Glucose concentrations were measured using the glucose oxidase method (Beckman Instruments, Fullerton CA). A subset of subjects (n = 112) spent 23 hours in a metabolic chamber on day 7 to determine respiratory quotient and 24-hour energy expenditure (see below). Abdominal subcutaneous adipose tissue was removed from the periumbilical region by percutaneous needle biopsy under local anesthesia (1% lidocaine) on day 9 as previously described10 for in vitro cell lipolysis. A total of 243 subjects returned to our research unit for follow-up visits at which body weight and composition were assessed (mean follow-up 8.2 ± 5.5 years; minimum 6 months).
Measurement of Whole Body Substrate Oxidation and Energy Expenditure
Subjects entered the calorimeter after an overnight fast and received meals at 7:00 am, 11:00 am, 4:00 pm, and 7:00 pm that they were instructed to finish. The diet was calculated to be eucaloric at sedentary conditions for each subject based on their weight11. Energy balance was calculated as energy expenditure-energy intake after accounting for any returned food. The macronutrient content of the chamber diet was 20% protein, 50% carbohydrate, 30% fat. Differences between outgoing and ingoing air concentrations of CO2 and O2 were measured continuously and mean values recorded every 15 minutes over 23 hours. Average calculated CO2 production and O2 consumption was extrapolated to 24 hours. The extrapolated averages were used to calculate substrate oxidation and energy expenditure as previously described12. Subject movement while in the chamber was continuously measured by a radar system.
In Vitro Methods
Osmium tetroxide fixed adipocytes’ sizes were determined with a Coulter Electronics counter with a 400-μm aperture equipped with a logarithmic range-expander and channelyzer (Coulter Electronics, Irvine, CA).
To determine basal and isoproterenol stimulated lipolysis, isolated adipocytes were incubated in a 500 μL 5% albumin-HEPES buffer at 37 °C for two hours with continuous shaking at 40 cycles/min. The buffer contained no isoproterenol (basal) or 25 nmol/L isoproterenol and 0, 12.5, 25, 50, 100, 200, or 8,000 pmol/L insulin. The incubation period was terminated by separating the cells from the medium by the oil method13. Glycerol in the incubation medium under the oil was determined by an enzymatic assay as previously described14.
Statistics
Baseline demographics were described using means and standard deviations for data that were normally distributed. Skewed data were reported as medians and interquartile ranges in the baseline demographics. Means from different subgroups were compared with ANOVA and Tukey’s HSD post-hoc test, skewed data from different subgroups with the Kruskal-Wallis test, and proportions from different subgroups with chi-square and Fisher’s exact test. Univariate associations between variables were calculated with Pearson product-moment correlations. Univariate associations between follow-up weight, fat mass, and fat free mass gain with average adipocyte size and in vitro basal and isoproterenol stimulated lipolysis used rate of percent gain, which was calculated with the following equation: [(Δkg/kgbl)*100]/Δyrs, where: Δkg = kg measurement change since baseline, kgbl = kg measurement at baseline, and Δyrs = years since the baseline measurement. Significant univariate associations were confirmed with multivariate general linear models. Multivariate models for whole body substrate oxidation in the metabolic chamber included fat mass, fat free mass, age, sex, and energy balance, and models for respiratory quotient included % body fat rather than fat mass and fat free mass15. Multivariate models for rate of weight, fat mass, or fat free mass gain during follow-up used rate of absolute change and were adjusted for age, sex, ethnicity, and initial weight, fat mass, or fat free mass, respectively. Basal lipolysis per cell and rate of fat mass change were log transformed to meet the assumptions of linear regression. Weight-change was assessed between sexes and ethnicities using t-tests. Analyses were performed using SAS 9.2 and SAS Enterprise Guide 4.2 (Cary, NC).
Results
Demographics and Subject Characteristics
Demographic and metabolic data for all analyses are shown in table 1. The majority of subjects (84%) in all analyses were Native American, and the average body composition (32.7% body fat) was considered overweight16. In vitro lipolysis measurements did not differ significantly between the various subgroups.
Table 1.
Demographic Information Table 1 compares the characteristics of all subjects who received an adipose tissue biopsy to the subgroups who also had follow-up data available and who had measurement of energy expenditure and substrate oxidation in a metabolic chamber
| Analysis | Units | Cross-Sectional | Follow-Up | Chamber | |||
|---|---|---|---|---|---|---|---|
| Gender | - | Male | Female | Male | Female | Male | Female |
| Race | NA/C/Ha | 142/36/1f | 130/16/0g | 115/11/1f | 114/2/0g | 33/26/0f | 46/7/0g |
| Age | Years (Mean±SDj) |
26.8±5.9 | 26.7±6.2 | 26.0±5.6 | 25.9±5.6 | 28.1±5.8 | 27.0±6.3 |
| Weight (kg) | Kg ( Mean±SDj ) |
100.3±27.8 | 90.5±21.6 | 102.3±28.4 | 91.3±20.8 | 106.0±29.9 | 93.6±22.7 |
| %Fat | % (Mean±SDj ) |
28.0±8.4 | 38.5±6.6 | 29.8±8.2 | 39.2±6.2 | 28.2±9.2 | 39.0±6.8 |
| Fx Glucoseb | mg/dl (Mean±SDj ) |
95.0±12.0 | 102.5±26.8 | 93.9±14.2 | 101.6±25.7 | 97.6±7.9 | 99.2±10.9 |
| 2H Glucoseb | mg/dl (Mean±SDj ) |
126.8±39.9 | 156.0±57.0 | 126.2±44.7 | 156.6±58.1 | 128.7±30.7 | 154.4±40.9 |
| BasLipc | fmoles/cell*s (Median[95% CIk]) |
.11[.07-.17] | .12[.08-.19] | .11[.08-.16] | .14[.08-.20] | .10[.07-.16] | .17[.08-.22] |
| BasLipSAd | 10-21
moles/μm2*s (Median[95% CIk]) |
2.5[1.7-3.7] | 2.8[1.8-4.0] | 2.7[1.8-3.7] | 3.0[1.9-4.3] | 2.4[1.8-3.4] | 2.9[1.4-4.2] |
| AVCSe | μg lipid/cell (Mean±SDj ) |
0.73±0.23h | 0.88±0.24i | 0.70±0.19h | 0.85±0.24i | 0.87±0.30h | 1.02±0.27i |
NA=Native American, C=Caucasian, H=Hispanic
Fx=Fasting and 2h=2 hour glucose (mg/dl)
BasLip=Basal lipolysis per cell
BasLipSA=Basal lipolysis per μm2
AVCS=Average cell size (μg lipid/cell)
Significant differences between all groups, p<0.001
Subjects with follow-up data different from all other groups, p=0.005
Subjects with data from the metabolic chamber different from all other groups, p<0.001
Subjects with data from the metabolic chamber different from all other groups, p<0.001
SD=Standard deviation
CI=Confidence interval
In Vitro Lipolysis is Related to Baseline Body Composition
There was a strong positive relationship between average adipocyte size (r=0.49, p<0.0001), basal (r=0.47, p<0.0001) and isoproterenol-stimulated (r=0.25, p<0.0001) lipolysis per cell with % body fat. Multivariate analysis showed that basal (p<0.0001), but not isoproterenol stimulated (p=0.80) lipolysis per cell, was associated with % body fat after accounting for adipocyte size as measured in μg lipid/cell (table 2).
Table 2.
Summary of Major Multivariate Analyses Models were assessed with and without average adipocyte cell size. Parameter estimates for sex are the differences associated with male vs. female sex and parameter estimates for ethnicity are the differences associated with Caucasian vs. Native American ethnicity
| Without adipocyte cell size | With adipocyte cell size | ||||
|---|---|---|---|---|---|
| Independent variable |
Dependent variable |
p value | Parameter estimate [95% CIe] |
p value | Parameter estimate [95% CIe] |
| BasLipa | %Fat | <0.0001 | 12.50 [10.4, 14.7] | <0.0001 | 7.9 [5.7, 10.2] |
| IpLipa | %Fat | <0.0001 | 8.70 [6.3, 11.0] | 0.80 | 0.3 [−2.3, 3.0] |
| Sex | BasLipa | <0.0001 | 0.11 [0.06, 0.17] | <0.0001 | 0.12 [0.07, 0.18] |
| %Fat | <0.0001 | 0.02 [0.02, 0.02] | <0.0001 | 0.01 [0.01, 0.02] | |
| AVCSb | BasLipa | <0.001 | 0.56 [0.46, 0.66] | - | - |
| Age | 0.30 | 0.01 [−0.00, 0.01] | - | - | |
| Sex | 0.63 | 0.01 [−0.04, 0.06] | - | - | |
| AVCSb | 24hRQ | 0.37 | −0.010 [−0.031, 0.012] | - | - |
| Age | 0.71 | 0.000 [−0.001, 0.001] | - | - | |
| EnBalc | 0.03 | 0.000 [0.000, 0.000] | - | - | |
| Sex | 0.43 | 0.005 [−0.008, 0.018] | - | - | |
| %Fat | 0.29 | 0.000 [−0.001, 0.000] | - | - | |
| AVCSb | Lipid oxidation |
0.44 | 72.1 [−112.6, 256.7] | - | - |
| Fat mass | 0.05 | 4.67 [−0.06, 9.40] | - | - | |
| FF massd | 0.09 | 5.30 [−0.92, 11.51] | - | - | |
| Age | 0.82 | 0.81 [−6.07, 7.70] | - | - | |
| Sex | 0.76 | 25.0 [−135.7, 185.8] | - | - | |
| EnBalc | <0.0001 | −0.54 [−0.74, −0.35] | - | - | |
| BasLipa | 24hRQ | <0.01 | −0.016 [−0.024, −0.008] | <0.01 | −0.017 [−0.025, −0.009] |
| Age | 0.56 | 0.000 [−0.001, 0.001] | 0.56 | 0.000 [−0.001, 0.001] | |
| EnBalc | 0.07 | 0.000 [−0.000, 0.000] | 0.07 | 0.000 [−0.000, 0.000] | |
| Sex | 0.50 | −0.005 [−0.018, 0.009] | 0.50 | −0.005 [−0.018, 0.009] | |
| %Fat | 0.33 | 0.000 [−0.001, 0.000] | 0.38 | 0.000 [−0.001, 0.000] | |
| BasLipa | Lipid oxidation |
0.04 | 164.8 [5.7, 323.9] | 0.04 | 186.1 [3.0, 369.1] |
| Fat mass | 0.06 | 4.61 [−0.24, 9.47] | 0.06 | 4.85 [−0.12, 9.83] | |
| FF massd | 0.17 | 4.52 [−1.90, 10.94] | 0.16 | 4.67 [−1.80, 11.15] | |
| Age | 0.52 | 2.22 [−4.68, 9.12] | 0.47 | 2.62 [−4.50, 9.74] | |
| Sex | 0.56 | 47.8 [−113.4, 208.9] | 0.63 | 39.9 [−125.2, 205.0] | |
| EnBalc | <0.0001 | −0.52 [−0.72, −0.32] | <0.0001 | −0.52 [−0.73, −0.32] | |
| AVCSb | Rate of fat mass change |
0.01 | −0.95 [−1.28, −0.62] | - | - |
| Fat mass | 0.53 | −0.01 [−0.03, 0.01] | - | - | |
| Ethnicity | <0.01 | 2.96 [1.41, 4.51] | - | - | |
| Sex | 0.62 | 0.08 [−1.54, 1.70] | - | - | |
| Age | 0.37 | 0.28 [−0.08, 0.64] | - | - | |
| BasLipa | Rate of weight change |
0.02 | −1.49 [−2.85, −0.15] | 0.03 | −1.53 [−2.95, −0.11] |
| Weight | 0.01 | 0.02 [0.00, 0.04] | 0.01 | 0.02 [0.00, 0.04] | |
| Ethnicity | 0.53 | 0.57 [−1.25, 2.39] | 0.53 | 0.57 [−1.25, 2.40] | |
| Sex | 0.78 | −0.11 [−0.84, 0.63] | 0.82 | −0.09 [−0.88, 0.70] | |
| Age | 0.39 | −0.03 [−0.09, 0.04] | 0.39 | −0.03 [−0.09, 0.04] | |
| BasLipa | Rate of fat mass change |
0.08 | −0.97 [−2.04, 0.10] | 0.06 | −1.11 [−2.24, 0.01] |
| Fat mass | 0.97 | 0.00 [−0.02, 0.02] | 0.87 | 0.00 [−0.02, 0.02] | |
| Ethnicity | 0.47 | 0.58 [−0.85, 2.01] | 0.44 | 0.56 [−0.87, 1.99] | |
| Sex | 0.49 | 0.25 [−0.81, 0.31] | 0.55 | −0.18 [−0.76, 0.41] | |
| Age | 0.26 | −0.03 [−0.08, 0.03] | 0.32 | −0.03 [−0.08, 0.03] | |
BasLip=Basal lipolysis and IpLip=isoproterenol stimulated lipolysis
AVCS=Average cell size
EnBal=Energy balance
FF mass=Fat free mass
95% CI=95% confidence interval
There was no difference in average adipocyte size, or basal or isoproterenol stimulated lipolysis between ethnicities (all p>0.05). Age was positively associated with average adipocyte size (r=0.18, p=0.0008), and this remained true after adjusting for % body fat (p=0.02). Age was not associated with basal (p=0.12) or isoproterenol stimulated (p=0.07) lipolysis. There was increased basal lipolysis per cell (p<0.0001; difference between least mean square means=0.03 fmoles/cell*s) in men only after adjusting for their comparatively lower % body fat (without adjustment for body fat females had higher basal lipolysis by 1.19 fmoles/cell*s). There was no interaction between sex and % body fat. Addition of adipocyte size to the model did not diminish the association between sex and basal lipolysis per cell (p<0.0001; table 2). Average adipocyte size and isoproterenol stimulated lipolysis did not differ between genders (both p>0.05).
In Vitro Lipolysis is Related to Adipocyte Size
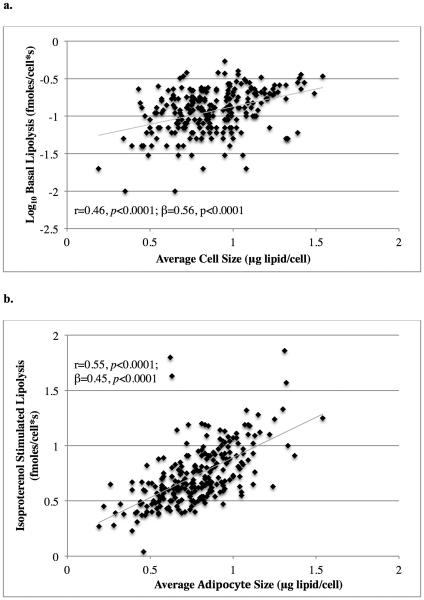
Average adipocyte size was strongly associated with basal lipolysis per cell (r=0.46, p<0.0001, figure 1a) and remained significant in multivariate models adjusted for age and sex (p<0.0001; β=0.56; table 2). Average adipocyte size was also associated with isoproterenol stimulated lipolysis per cell (r=0.55, p<0.0001, figure 1b), again remaining significant in multivariate models adjusted for age and sex (p<0.0001; β=0.45). Basal lipolysis was strongly associated with ED50 for insulin’s antilipolytic action (r=0.33, p<0.001).
Figure 1. Association Between Adipocyte Size and Lipolysis.
The association between average adipocyte size and basal lipolysis per cell (a.) and isoproterenol stimulated lipolysis per cell (b.). Basal lipolysis values are shown on a logarithmic scale. Unadjusted Pearson product-moment correlations (r) and adjusted parameter estimates (β) are reported with their associated p values.
Whole body Substrate Oxidation is Associated with In Vitro Lipolysis
Average adipocyte size was negatively associated with 24-hour RQ (24hRQ; r=−0.22, p=0.01), although this was no longer significant after adjustment for co-variates (p=0.37; table 2). Its association with whole body lipid oxidation (r=0.34, p=0.0001) was also significantly reduced in multivariate models (p=0.44; table 2). Average adipocyte size was not associated with whole body carbohydrate oxidation (r=0.09, p=0.30).
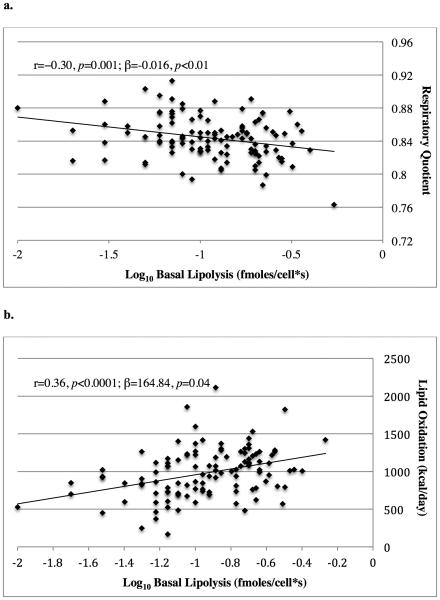
Basal lipolysis per cell was negatively associated with 24hRQ (r=−0.30, p=0.001) and positively correlated with whole body lipid oxidation (r=0.36, p<0.0001; figure 2). These associations remained significant after adjustment for co-variates (p<0.01 and p=0.04, respectively; table 2). Including average adipocyte size in the models did not reduce the association between basal lipolysis per cell and 24hRQ (p<0.01) or whole body lipid oxidation (p=0.04; table 2). Basal lipolysis per cell was not associated with carbohydrate oxidation (r=0.04, p=0.62).
Figure 2. Association Between In Vitro Lipolysis and Whole Body Substrate Oxidation.
The association between basal lipolysis per cell with respiratory quotient (a.) and lipid oxidation (b.) during a 24-hour stay in a calorimeter. Basal lipolysis values are shown on a logarithmic scale. Unadjusted Pearson product-moment correlations (r) and adjusted parameter estimates (β) are reported with their associated p values.
There was no association between isoproterenol stimulated lipolysis and whole body lipid (r=0.01, p=0.22) or carbohydrate (r=−0.05, p=0.62) oxidation, or 24hRQ (r=−0.08, p=0.17). Neither average adipocyte size nor any measurement of in vitro lipolysis was associated with 24-hour energy expenditure (all p>0.05). ED50 for insulin’s antilipolytic activity was not associated with any measurement of substrate oxidation.
Relationship Between In Vitro Lipolysis and Future Weight Gain
Subjects gained 8.5 ± 15.0 kg during the follow-up period (p<0.0001), or 9.3 ± 15.5% of their original body weight. Neither weight change nor rate of weight gain varied by gender. An average of 54% of the weight gained during follow-up among all subjects was fat mass. This did not differ between ethnicities or genders.
Average adipocyte size was not associated with rate of percent weight (r=−0.08, p=0.20) or fat free mass (r=−0.04, p=0.76) gain. It was, though, negatively associated with rate of percent fat mass gain (r=−0.16, p=0.02) and remained significant after adjustment for co-variates (p=0.01; table 2).
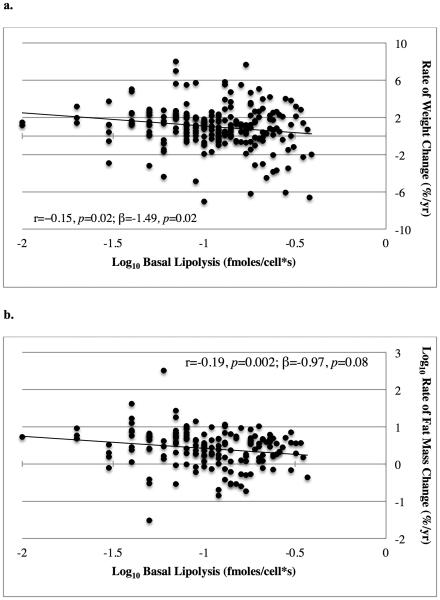
Basal lipolysis per cell was negatively associated with rate of percent weight gain (r=−0.15, p=0.02, figure 3a). This association remained significant in multivariate models adjusting for age, sex, and ethnicity (p=0.02; β=−1.49; table 2). There was no significant interaction between sex and basal lipolysis in this association (p=0.33). Addition of average adipocyte size to the multivariate model slightly attenuated the negative association between basal lipolysis per cell and rate of percent weight gain (p<0.05; β=−1.53; table 2), indicating that this association may be due in part to larger adipocytes releasing more lipid. However, average adipocyte size itself was not significantly associated with weight gain in this model (p=0.63). There was a negative association between basal lipolysis per cell and rate of percent fat mass gain (r=−0.19, p=0.002, figure 3b), which was attenuated in the multivariate models (p=0.08; β=−0.97) and after added adjustment for average adipocyte size (p=0.06; β=−1.11; table 2). Basal lipolysis measurements were not associated with rate of fat free mass change during the follow-up period (r=0.00, p=0.96). Additionally, there was no association between isoproterenol stimulated lipolysis per cell and rate of percent weight, fat mass, or fat free mass change during follow-up (all p>0.05). ED50 for insulin’s antilipolytic action was not an independent predictor of future rate of percent weight, fat mass, or fat free mass change during follow-up after adjusting for basal lipolysis (all p>0.05). To normalize for glycerol release, we expressed basal lipolysis as fmol/s and included cell size (measured in μg lipid/cell) in the models, which did not alter the results (data not shown).
Figure 3. Association Between In Vitro Lipolysis and Weight Gain.
The association between basal lipolysis per cell with rate of percent weight change (a.) and rate of percent fat mass change (b.) Lipolysis values and rate of percent fat mass change are shown on logarithmic scales. Unadjusted Pearson product-moment correlations (r) and adjusted parameter estimates (β) are reported with their associated p values.
Discussion
We found that percent body fat was positively associated with average adipocyte size and in vitro basal lipolysis. In vitro basal lipolysis was positively associated with whole body lipid oxidation and negatively with 24-hour respiratory quotient. Larger adipocyte size and higher basal lipolysis predicted lower fat mass gain in univariate models. However, the association between basal lipolysis and fat mass gain was marginal after accounting for known predictors of fat mass gain. In multivariate models higher basal lipolysis but not adipocyte size was associated with lower total weight gain. Neither adipocyte size nor basal lipolysis predicted fat free mass gain. Maximal isoproterenol stimulated lipolysis was not associated with weight, fat mass, or fat free mass gain.
Weyer and colleagues previously observed a positive association between average adipocyte size and basal lipolysis per cell in a smaller sample of Pima Indians17. Larger adipocytes have been shown to have enhanced lipolytic capacity compared to smaller adipocytes in healthy human subject of European ancestry18. Elevated basal lipolysis has also been seen in large compared to small adipocytes from C57BL/6J mice fed a high fat diet19. In our large cohort, we confirmed an association between adipocyte size and both basal and isoproterenol stimulated in vitro lipolysis in a large cohort.
We investigated whether in vitro cellular lipolysis was associated with whole body substrate oxidation to establish a possible mechanistic link through which adipocyte lipolysis could affect body weight. Imbeult and colleagues found that in vitro isoproterenol stimulated lipolysis was associated with whole body oxidation in a study of 20 obese Caucasian males20. However, substrate oxidation in that study was measured for 15 minutes in an exclusively fasting state, which may not reflect free-living substrate oxidation. Moro and colleagues performed in vitro microdialysis measurement lipolysis in 8 endurance-trained male cyclists while measuring substrate oxidation during exercise21. Like the previous study, this method may not produce findings generalizable to the public in typical conditions. Studies have also shown that in vitro cellular lipolysis, whole body lipolysis, and whole body lipid oxidation shift similarly after a high fat diet. Kather and colleagues performed in vitro cell lipolysis on adipose tissue after feeding subjects different diets and found that basal lipolysis was increased after a high fat diet or an energy restricted diet22 and Calles-Escandón and Driscoll found that fat intake was positively correlated with in vivo basal lipolysis in humans23. These observed associations indicate a link between in vitro measurements of lipolysis and whole body substrate oxidation. The setting of our study was unique as subjects were fed a weight-maintaining diet with standardized macronutrient composition throughout their inpatient stay providing time prior to any studies to allow cellular and whole body measurements for fat oxidation to adapt from free-living conditions24. Under these conditions, we found in vitro basal lipolysis per cell was associated with 24-hour RQ and lipid oxidation, but not carbohydrate oxidation. Thus, we were able to provide evidence that an in vitro cellular measurement may reflect whole body lipid metabolism. Average adipocyte size was also negatively associated with RQ and positively with lipid oxidation, although these associations were not statistically significant when assessed in multivariate models.
We then investigated whether average adipocyte size and in vitro lipolysis were associated with weight gain. Landerholm and Stern had found a negative association between baseline epinephrine stimulated lipolysis and weight gain in Sprague-Dawley rats fed a high-fat diet25. We demonstrated that in humans in vitro basal lipolysis independent of adipocyte cell size was negatively associated with total weight gain. Andersson, Wahrenberg, and Löfgren found an association between CGP 12177, a β3-adrenoreceptor agonist, stimulated lipolysis and weight gain in female humans26. However, we did not find an association between lipolysis stimulated by isoproterenol, which has similar β-selectivity to epinephrine, and weight gain in our mixed sex cohort. We found that adipocyte size and in vitro basal lipolysis were negatively associated with fat mass gain, although the association between in vitro basal lipolysis and fat mass gain was attenuated when assessed in a multivariate model. Our in vitro results (given the associations with RQ and lipid oxidation) are consistent with previous studies. We have shown that higher respiratory quotient, and, in men, lower lipid oxidation predicted weight gain4,27. Froidevaux et al also demonstrated that an elevated RQ following a dietary intervention increased risk of regaining weight in women5. Our results indicate a link between cellular lipolysis and weight gain via whole body lipid oxidation.
Stored triglycerides must go through lipolysis before the body can utilize them for energy. Lipolysis increases with fasting and calorie deficit, conditions that cause weight loss and utilization of energy stores22,28,29. We demonstrated that even during a macronutrient stable weight maintaining diet prior to adipose tissue biopsy and calorimetry measurements, that higher in vitro lipolytic rates are associated with whole body lipid oxidation and lower rates of weight gain. Individuals whose adipocytes have increased basal lipolysis in the eucaloric state may preferentially use lipids for energy rather than store them allowing for increased lipid oxidation rates and hence lower rates of weight and perhaps fat mass gain. Additionally, increased lipid oxidation may divert carbohydrates to glycogen, which, in the free-living environment, may reduce food intake15,30.
Several limitations of this study must be acknowledged. First, a majority of subjects were Pima Indian, which may limit the generalizability of the findings. However, there was no observed difference in cellular basal lipolysis between ethnicities. Second, not all subjects completed measurements of substrate oxidation and follow-up body composition. Analysis of these variables, though, was done independently and differences in the demographics of the subgroups were accounted for. Additionally, a determination of causality in the relationship between in vitro cellular basal lipolysis and weight change cannot be made with these data.
Increased adipocyte cell size and in vitro lipolysis were positively associated. We further demonstrated that in vitro lipolysis independent of co-variates including adipocyte cell size was associated with RQ and whole body lipid oxidation, both previously identified metabolic predictors of weight gain. Higher basal lipolysis was also associated with less weight gain, again independent of cell size. Adipocyte size and basal lipolysis may be inherent characteristics of individual adipose cells that have whole body effects on both lipid oxidation and eventual weight gain.
Study Importance.
What is Already Known About This Subject
In vitro cellular lipolysis and whole body lipid oxidation shift similarly after a change in diet, and the two measures have been found to be associated in a small study of obese men
Whole body lipid oxidation has been shown to be associated with future weight gain in Native American men and Caucasian women, suggesting in vitro lipolysis may be associated with weight gain
What This Study Adds
We found that in vitro basal lipolysis had a positive association with whole body lipid oxidation in a large, mixed-weight sample of men and women
We found that in vitro basal lipolysis had a negative association with weight gain and a marginal negative association with fat mass gain in a mixed sex and race cohort
Acknowledgements
The authors thank the volunteers who participated in the studies. They also thank the clinical staff of the Phoenix Epidemiology and Clinical Research Branch for conducting the examinations.
Funding: This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. doi:10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 2.Michaud A, Boulet MM, Veilleux A, Noël S, Paris G, Tchernof A. Abdominal subcutaneous and omental adipocyte morphology and its relation to gene expression, lipolysis and adipocytokine levels in women. Metabolism. 2014;63(3):372–381. doi: 10.1016/j.metabol.2013.11.007. doi:10.1016/j.metabol.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Schutz Y, Flatt JP, Jéquier E. Failure of dietary fat intake to promote fat oxidation: a factor favoring the development of obesity. Am J Clin Nutr. 1989;50(2):307–314. doi: 10.1093/ajcn/50.2.307. [DOI] [PubMed] [Google Scholar]
- 4.Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab. 2013;98(4):E703–E707. doi: 10.1210/jc.2012-3529. doi:10.1210/jc.2012-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froidevaux F, Schutz Y, Christin L, Jéquier E. Energy expenditure in obese women before and during weight loss, after refeeding, and in the weight-relapse period. Am J Clin Nutr. 1993;57(1):35–42. doi: 10.1093/ajcn/57.1.35. [DOI] [PubMed] [Google Scholar]
- 6.Seidell JC, Muller DC, Sorkin JD, Andres R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 1992;16(9):667–674. [PubMed] [Google Scholar]
- 7.Weyer C, Snitker S, Rising R, Bogardus C, Ravussin E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. Int J Obes. 1999;23(7):715–722. doi: 10.1038/sj.ijo.0800910. doi:10.1038/sj.ijo.0800910. [DOI] [PubMed] [Google Scholar]
- 8.Ravussin E, Gautier JF. Metabolic predictors of weight gain. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 1999;23(Suppl 1):37–41. doi: 10.1038/sj.ijo.0800793. [DOI] [PubMed] [Google Scholar]
- 9.Keys A, BroŽek J. Body Fat in Adult Man. Physiol Rev. 1953;33(3):245–325. doi: 10.1152/physrev.1953.33.3.245. [DOI] [PubMed] [Google Scholar]
- 10.Koska J, Stefan N, Permana PA, Weyer C, Sonoda M, Bogardus C, Smith SR, Joanisse DR, Funahashi T, Krakoff J, Bunt JC. Increased fat accumulation in liver may link insulin resistance with subcutaneous abdominal adipocyte enlargement, visceral adiposity, and hypoadiponectinemia in obese individuals. Am J Clin Nutr. 2008;87(2):295–302. doi: 10.1093/ajcn/87.2.295. [DOI] [PubMed] [Google Scholar]
- 11.Abbott WG, Howard BV, Christin L, Freymond D, Lillioja S, Boyce VL, Anderson TE, Bogardus C, Ravussin E. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. Am J Physiol. 1988;255(3):E332–E337. doi: 10.1152/ajpendo.1988.255.3.E332. Pt 1. [DOI] [PubMed] [Google Scholar]
- 12.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78(6):1568–1578. doi: 10.1172/JCI112749. doi:10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gliemann J, Sonne O. Binding and receptor-mediated degradation of insulin in adipocytes. J Biol Chem. 1978;253(21):7857–7863. [PubMed] [Google Scholar]
- 14.Bergmeyer H-Ui. Methods of Enzymatic Analysis. Elsevier; 2012. [Google Scholar]
- 15.Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007;86(3):625–632. doi: 10.1093/ajcn/86.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72(3):694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 17.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43(12):1498–1506. doi: 10.1007/s001250051560. doi:10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 18.Laurencikiene J, Skurk T, Kulyté A, Hedén P, Aström G, Sjölin E, Rydén M, Hauner H, Arner P. Regulation of lipolysis in small and large fat cells of the same subject. J Clin Endocrinol Metab. 2011;96(12):E2045–E2049. doi: 10.1210/jc.2011-1702. doi:10.1210/jc.2011-1702. [DOI] [PubMed] [Google Scholar]
- 19.Wueest S, Rapold RA, Rytka JM, Schoenle EJ, Konrad D. Basal lipolysis, not the degree of insulin resistance, differentiates large from small isolated adipocytes in high-fat fed mice. Diabetologia. 2009;52(3):541–546. doi: 10.1007/s00125-008-1223-5. doi:10.1007/s00125-008-1223-5. [DOI] [PubMed] [Google Scholar]
- 20.Imbeault P, Tremblay A, Després J, Mauriège P. beta-adrenoceptor-stimulated lipolysis of subcutaneous abdominal adipocytes as a determinant of fat oxidation in obese men. Eur J Clin Invest. 2000;30(4):290–296. doi: 10.1046/j.1365-2362.2000.00634.x. [DOI] [PubMed] [Google Scholar]
- 21.Moro C, Harant I, Badin P-M, Patarca F-X, Guilland J-C, Bourlier V, Langin D, De Glisezinski I. Influence of lipolysis and fatty acid availability on fuel selection during exercise. J Physiol Biochem. 2014;70(2):583–591. doi: 10.1007/s13105-013-0306-z. doi:10.1007/s13105-013-0306-z. [DOI] [PubMed] [Google Scholar]
- 22.Kather H, Wieland E, Scheurer A, Vogel G, Wildenberg U, Joost C. Influences of variation in total energy intake and dietary composition on regulation of fat cell lipolysis in ideal-weight subjects. J Clin Invest. 1987;80(2):566–572. doi: 10.1172/JCI113105. doi:10.1172/JCI113105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calles-Escandón J, Driscoll P. Diet and body composition as determinants of basal lipolysis in humans. Am J Clin Nutr. 1995;61(3):543–548. doi: 10.1093/ajcn/61.3.543. [DOI] [PubMed] [Google Scholar]
- 24.Schrauwen P, van M Lichtenbelt WD, Saris WH, Westerterp KR. Changes in fat oxidation in response to a high-fat diet. Am J Clin Nutr. 1997;66(2):276–282. doi: 10.1093/ajcn/66.2.276. [DOI] [PubMed] [Google Scholar]
- 25.Landerholm TE, Stern JS. Adipose tissue lipolysis in vitro: a predictor of diet-induced obesity in female rats. Am J Physiol. 1992;263(6):R1248–R1253. doi: 10.1152/ajpregu.1992.263.6.R1248. Pt 2. [DOI] [PubMed] [Google Scholar]
- 26.Andersson D, Wahrenberg H, Löfgren P. Beta3-adrenoceptor function and long-term changes in body weight. Int J Obes 2005. 2009;33(6):662–668. doi: 10.1038/ijo.2009.54. doi:10.1038/ijo.2009.54. [DOI] [PubMed] [Google Scholar]
- 27.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, Swinburn BA, Knowler WC, Bogardus C, Ravussin E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259(5):E650–E657. doi: 10.1152/ajpendo.1990.259.5.E650. Pt 1. [DOI] [PubMed] [Google Scholar]
- 28.Jensen MD, Haymond MW, Gerich JE, Cryer PE, Miles JM. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J Clin Invest. 1987;79(1):207–213. doi: 10.1172/JCI112785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arner P. Human fat cell lipolysis: Biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005;19(4):471–482. doi: 10.1016/j.beem.2005.07.004. doi:10.1016/j.beem.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Flatt JP. The difference in the storage capacities for carbohydrate and for fat, and its implications in the regulation of body weight. Ann N Y Acad Sci. 1987;499:104–123. doi: 10.1111/j.1749-6632.1987.tb36202.x. [DOI] [PubMed] [Google Scholar]