Abstract
Objective
Traumatic stressors, including child abuse and/or interpersonal violence over a woman’s lifecourse, can affect the health of her children. This study examines associations between maternal lifetime interpersonal trauma (IPT) and children’s asthma by age six years (N=857).
Methods
Pregnant women completed the Revised Conflict Tactics Scale; IPT exposure was categorized as unexposed (55%), early (childhood and/or teen years only, 25%), late (adulthood and/or index pregnancy, 7%), and chronic (early and late, 13%). Clinician-diagnosed asthma in children was reported by mothers at each follow-up visit until the child reached age 6 years. We examined effects of maternal IPT categories and child’s asthma using logistic regression. Using structural equation models, we also examined indirect relationships between maternal chronic IPT and child asthma operating through active asthma in pregnancy, pre-pregnancy BMI, prenatal smoking, and/or increased exposure to other adverse life events or environmental toxins prenatally. Effect modification by the child’s sex was examined.
Results
Mothers were primarily Hispanic (55%) or Black (30%) with < high school education (62%). In logistic regression models, chronic maternal IPT (compared to unexposed) was associated with asthma in boys (OR=2.87; 95% CI, 1.48–5.57) but not girls (OR=0.69; 95% CI 0.23–2.12) (pinteraction=0.042). In SEMs, chronic IPT was associated with maternal active asthma in pregnancy (β=0.59, p<0.001); maternal active asthma was associated with children’s asthma (β=0.20, p=0.009); and the total indirect effect for this path was significant (β=0.12, p=0.031). Associations were most evident among boys.
Conclusions
Mothers’ history of chronic interpersonal trauma was associated with asthma in boys. This association was mediated through active maternal asthma in pregnancy.
Keywords: Pediatric asthma, interpersonal trauma, minorities, pregnancy, sex differences
INTRODUCTION
Children born to mothers experiencing higher maternal psychosocial stress in pregnancy are at increased risk of developing asthma. (1) Stressors are largely thought to affect pathogenesis by initiating dysregulated biobehavioral states resulting in lasting effects on key physiological processes that influence disease risk.(2–4) As a response to stress, normal homeostatic functions of the body’s key physiological systems are disrupted; the disturbed balance of these systems is most relevant to disease. For instance, a defensive biological response by the neural, immune, and endocrine systems is important for the short-term stress response; however, this type of response may produce long-term damage if not checked and terminated.(5) Although the disturbance of the hypothalamic-pituitary-adrenal (HPA) axis is most widely studied, research suggests that autonomic imbalance or dysfunction, independent of hormonal or neuroendocrine abnormalities may be just as important.(6–8) Both animal and human data suggest that the disruption of these key stress systems in the mother resulting from her own stress history (9–11) can influence immune functioning and other mechanisms in the developing fetus that in turn have been linked to asthma development.(12, 13) For example, biomarker correlates of disrupted stress-response systems in pregnant women, such as cortisol, have been linked to early respiratory outcomes in children (14, 15). Increased prenatal stress and cortisol have also been associated with immune profiles [e.g., T-helper (Th) 2 response, Th17 pathway promotion] at birth related to increased childhood asthma risk.(16–18).
Existing studies examining links between prenatal maternal stress and child asthma have considered stress occurring proximate to the index pregnancy through current negative life events (19, 20) or prenatal psychological functioning (e.g., anxiety).(21) However, evolving research points to the need for a broader view that considers stress experienced over the mother’s lifecourse when characterizing risk to the next generation. A large body of literature links interpersonal trauma (IPT), including child abuse and/or interpersonal violence experienced across the lifecourse, to a range of psychological and physical health sequelae among women. Chronic stress associated with IPT has been associated with physiologic correlates of stress in pregnant women long after the exposure including altered cortisol levels, (22, 23) autonomic imbalance,(24) immune disruption and chronic inflammation.(25, 26) Notably, women experiencing traumatic events such as abuse and neglect in childhood/adolescence (25) or intimate partner violence (26) also exhibit elevated levels of C-reactive protein (CRP), a marker of systemic inflammation. A recent study linked elevated CRP in pregnancy to increased asthma risk in children by age 3 years.(27) Thus, evidence shows that IPT over a woman’s lifecourse can have lasting effects on key stress-response systems during pregnancy; however, studies examining associations between lifetime maternal trauma and child health are sparse. This is an important consideration in order to comprehensively understand the public health implications of maternal trauma.
Taking a lifecourse perspective, the timing of trauma exposure in the mother may be important. Exposures during sensitive developmental periods in the mothers, with both early childhood and adolescence identified as periods susceptible to traumatic stress effects,(28) or cumulative stress due to ongoing IPT over the lifetime may be particularly likely to affect psychophysiological functioning at childbearing age.(29) Thus, maternal IPT may be linked to fetal and infant health through more latent effects (i.e., lasting effects from childhood), proximate effects (i.e., trauma experienced in or around the pregnancy), or cumulative effects (i.e., traumas occurring over multiple periods in the mother’s lifecourse).
In addition to the direct programming effects on these physiological systems, maternal traumatic stress may operate through a number of intermediary pathways to indirectly influence asthma development in children. This hypothesis underscores the concept that lifetime trauma can lead to adverse behavioral and physical health consequences in women that in turn are known antecedents of adverse fetal development and asthma risk. For instance, our group and others have linked interpersonal violence in earlier life with current asthma in adult women.(30, 31); thus, trauma exposure may make it more likely for women to have persistent and active asthma at the time of pregnancy. Maternal active asthma during pregnancy, in turn, has been associated with increased asthma incidence in children.(32, 33) While genetic predisposition likely plays a role in childhood asthma risk, numerous studies have shown that maternal active asthma during pregnancy has a direct effect on fetal development and a child’s respiratory health through restricted oxygenation, dysfunction of bronchial relaxation, cytokine expression, and placental sensitivity to cortisol and intrauterine glucocorticoid exposure.(33, 34) Other studies link trauma histories to pre-pregnancy obesity (35) and smoking during pregnancy.(36) Both maternal obesity and prenatal smoking have been linked to childhood asthma.(37)
IPT experiences are more common in the general population than previously thought, particularly among lower-income, minority populations.(38, 39) Poverty is associated with increased exposure to traumatic stressors as well.(40) Lower-income women exposed to chronic IPT are also more likely to experience other adverse events (41) or be exposed to physical environmental factors associated with asthma such as air pollution. (42)
Leveraging a lower-income, ethnically diverse prospective birth cohort, we examined associations between lifetime IPT in women and asthma in their children and hypothesized that children born to women exposed to chronic IPT over their lifetime would be most likely to develop asthma. Structural equation modeling (SEM) was used to examine indirect pathways that may be operating between maternal IPT and children’s asthma development. Specifically, we examined whether the association between maternal IPT and offspring asthma operated through maternal active asthma in pregnancy, maternal pre-pregnancy obesity, an increased likelihood of maternal prenatal smoking, and/or increased exposure to other adverse events or environmental factors (air pollution) during pregnancy. Given evidence that prenatal stress may have sex-specific effects on child developmental outcomes,(43) associations were examined among boys and girls separately.
METHODS
Sample
Subjects were from the Asthma Coalition on Community Environment and Social Stress (ACCESS) project, a prospective pregnancy cohort designed to examine the independent and interactive effects of early-life psychosocial stress and physical toxins on childhood respiratory health.(44) Briefly, English or Spanish-speaking pregnant women (≥18 years old) receiving routine prenatal care at Brigham & Women’s Hospital (BWH), Boston Medical Center (BMC), and affiliated community health centers were enrolled at 28.4 ± 7.9 weeks gestation from August 2002 to December 2009. Among the women approached who met eligibility criteria, 989 (78.1%) agreed to enroll and 955 gave birth to a singleton live-born infant and continued follow-up. There were no significant differences in race/ethnicity, education, and income between women enrolled and those who declined.(45) Data on sociodemographics, maternal health, and prenatal exposures were obtained within two weeks of enrollment. Procedures were approved by the human studies committees at Brigham & Women’s Hospital and Boston Medical Center; written consent was obtained in the mother’s primary language.
Maternal Lifetime Interpersonal Trauma
Maternal report of lifetime IPT was assessed using the Revised Conflict Tactics Scale (R-CTS) short form; this scale has documented reliability (range, 0.79–0.95) and has been validated in English and Spanish.(46) Trauma was assessed during childhood (≤ 11 years old), adolescence (12–17 years old), adulthood before pregnancy, and during pregnancy. For each life stage, mothers reported whether anyone had ever pushed, grabbed, or shoved them; kicked, bit, or punched them; hit them with something that hurt their body; choked or burned them; forced them to have sexual activities; or physically attacked them in some other way. For the childhood period, the stem to the question was modified to ask, “Did anyone at least 5 years older than you ever…” followed by the items. Mothers were considered exposed in each period if they answered yes to any item. Women were categorized into groups defined as unexposed, early IPT (IPT experienced during childhood/adolescence only), late IPT (IPT experienced during adulthood/index pregnancy only), or chronic IPT (both periods).
Childhood Asthma Outcome
Maternal-reported clinician-diagnosed asthma was ascertained from birth up to age six years through telephone and in-person interviews at 3-month intervals for the first 24 months and annually thereafter. Mothers were asked the following: “Has a doctor or nurse ever said your child has asthma?” If a mother positively endorsed this question, the child was considered asthmatic. Most asthma diagnoses were made after the children were 3 years of age (78.6%).(45)
Pathway Variables
Maternal active asthma
Mothers were asked to report whether she was ever diagnosed with asthma by a healthcare provider. In the third trimester and again within 3 months postpartum, women were asked about asthma symptoms, use of asthma medications, and healthcare utilization for asthma (e.g., emergency room visits, hospitalizations) and when this had most recently occurred. A mother was considered to have asthma during pregnancy if she was ever diagnosed with asthma and she reported asthma symptoms (i.e., wheezing/whistling in her chest, nocturnal awakening with wheeze or cough), taking asthma medications including bronchodilators or steroids (inhaled/oral), or utilization of healthcare for asthma during the pregnancy.
Maternal body mass index
Maternal body mass index (BMI) was calculated by dividing weight by height squared (kg/m2) from pre-pregnancy weight and height reported by women. An internal validation study comparing self-reported and measured weight and height early in pregnancy (< 10 weeks) showed good agreement.(14)
Smoking during pregnancy
Mothers reported smoking at enrollment and in the third trimester; women were classified as prenatal smokers if smoking at either visit.
Prenatal negative life events
The Crisis in Family Systems-Revised (CRISYS) survey was administered in pregnancy within 2 weeks of enrollment to assess exposure to negative life events over the past six months. The CRISYS has good test/retest reliability, is suitable for lower-income populations and has been validated in English and Spanish.(47, 48) The survey assesses life events experienced across 11 domains (e.g., financial, legal, career, relationship stability, safety in the community/home, housing problems, difficulty with authority, discrimination), with multiple items in each domain. Women endorsed experiences as positive, negative, or neutral. The number of domains with one or more negative events was summed to create a negative life events (NLEs) domain score, with higher scores indicating greater stress. Women in this study reported experiencing negative events in none to a maximum of nine domains.
Prenatal ambient air pollution
Ambient particulate matter (PM2.5), a marker of traffic-related air pollution and other sources, was estimated based on resident address over the entire pregnancy. Residence-specific estimates of PM2.5 were determined by using a spatio-temporal model incorporating Moderate Resolution Imaging Spectroradiometer (MODIS) satellite-derived Aerosol Optical Depth (AOD) measurements at a 10 × 10 km spatial resolution and layering this remote sensing data with traditional Land-Use Regression (LUR) predictors as detailed previously.(45, 49)
Covariates
We also considered variables known to be associated with childhood asthma and/or maternal IPT including maternal age (years), maternal education (< high school or ≥ high school degree), race/ethnicity (Hispanic, Black, White, other), child’s sex, and child’s birthweight adjusted for gestational age (birthweight z-score).(50)
Statistical Analyses
Mother-child dyads (n=857) with complete data on IPT exposure and child asthma by age 6 years were included in these analyses. Those included vs. those excluded from these analyses (n=98) did not differ on key sociodemographic characteristics [85% vs. 87% Hispanic or African American, respectively (p=0.39); 62% vs. 66% with a high school education or less, respectively (p=0.94)].
Descriptive statistics, among those included in these analyses, were examined for the overall sample and then relative to child’s sex and asthma status. Differences in covariate distribution by child’s sex and asthma status were examined by using Kruskal-Wallis ANOVA and χ2 test to examine differences in means and proportions, respectively.
Logistic regression was used to test the relationship between maternal early, late, and chronic IPT (no IPT exposure as reference) and child asthma. Covariates examined for inclusion in logistic models were maternal education, maternal age, race/ethnicity, child’s sex, and birthweight z-score. Since birthweight z-score was not significantly related to maternal IPT or asthma, it was excluded from the logistic regression analyses. We examined associations in analyses stratified by child sex and tested the first-order interaction between maternal IPT and child’s sex by adding a product term to the model. Logistic regression analyses were conducted in SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Path analyses (a special case of structural equation modeling) were used to test the direct and indirect effects of maternal chronic IPT on the child’s risk for asthma. This allowed us to test whether associations between maternal chronic IPT and child’s asthma risk were in part mediated through active asthma in pregnancy, increased pre-pregnancy BMI, prenatal smoking, and/or increased exposure to other adverse life events or physical environmental toxins such as prenatal ambient air pollution. All path analyses were adjusted for child’s sex, race/ethnicity, maternal education, and maternal age. The birthweight z-score was significantly associated with some of the potential path variables (maternal pre-pregnancy BMI, smoking during pregnancy), thus this was also included as a covariate in path models. Path analyses were conducted for the entire cohort adjusted for child’s sex, race/ethnicity, maternal education, maternal age, and birthweight z-score; analyses considering boys and girls separately adjusted for all other covariates. These analyses were implemented in MPLUS.
RESULTS
Table 1 summarizes the distribution of covariates in the sample as a whole and stratified by child’s sex and asthma status. The average maternal age was 27.0 years; most were Hispanic (55%) or Black (30%) with less than a high school education (62%); 136 (16%) women reported having active asthma during pregnancy and 134 (15.6%) of children were diagnosed with asthma by age 6 years. Boys were more likely than girls to be diagnosed with asthma [18.7% (n=82) vs 12.4% (n=52) p=0.012)]. Children born to mothers with higher BMI (30.2 vs. 28.7, p=0.021) and active asthma in pregnancy (26% vs. 14%, p<0.001) were more likely to be diagnosed with asthma. There were more mothers exposed to chronic lifetime IPT (20% vs. 12%, p = 0.05) and other negative life events in pregnancy (2.9 vs. 2.4, p = 0.009) among children with asthma versus those without. The prevalence of maternal prenatal smoking was higher among asthmatic vs. nonasthmatic children (p=0.09).
Table 1.
Description of covariates and path variables by child’s sex and asthma status
| Child’s Sex
|
Child’s Asthma Status
|
||||||
|---|---|---|---|---|---|---|---|
| All (n=857) |
Boys (n=438) |
Girls (n=419) |
P-value | Yes (n=134) |
No (n=723) |
P-value | |
| Ever have asthma up to age 6 yrs [no. (%)] | 0.012 | ||||||
| No | 723 (84.4) | 356 (81.3) | 367 (87.6) | – | – | ||
| Yes | 134 (15.6) | 82 (18.7) | 52 (12.4) | – | – | ||
| Maternal Age [years; mean (± SD)] | 27.0 ± 5.9 | 27.2 ± 6.0 | 26.8 ± 5.9 | 0.34 | 26.5 ± 5.7 | 27.1 ± 6.0 | 0.39 |
| Pre-pregnancy BMI [ kg/m2 ; mean (± SD)] | 28.9 ± 6.4 | 28.8 ± 6.3 | 29.1 ± 6.6 | 0.63 | 30.2 ± 6.7 | 28.7 ± 6.4 | 0.021 |
| Education [no. (%)] | 0.93 | 0.34 | |||||
| < High School | 528 (62) | 144 (33) | 139 (33) | 78 (58) | 450 (62) | ||
| ≥ High School degree | 283 (33) | 267 (61) | 261 (62) | 49 (37) | 234 (32) | ||
| Missing | 46 (5) | 27 (6) | 19 (5) | 7 (5) | 39 (6) | ||
| Race/ethnicity [no. (%)] | 0.59 | 0.39 | |||||
| Hispanic | 471 (55) | 228 (52) | 243 (58) | 67 (50) | 404 (56) | ||
| Black | 257 (30) | 136 (31) | 121 (29) | 40 (30) | 217 (30) | ||
| White | 77 (9) | 43 (10) | 34 (8) | 13 (10) | 64 (9) | ||
| Other/mixed | 52 (6) | 31 (7) | 21 (5) | 14 (10) | 38 (5) | ||
| Lifetime IPT [no. (%)] | 0.15 | 0.05 | |||||
| Never exposed | 473 (55) | 230 (53) | 243 (58) | 65 (49) | 408 (56) | ||
| Early-life IPT | 215 (25) | 110 (25) | 105 (25) | 36 (27) | 179 (25) | ||
| Late/Current IPT | 55 (7) | 29 (7) | 26 (6) | 6 (4) | 49 (7) | ||
| Chronic IPT | 114 (13) | 69 (16) | 45 (11) | 27 (20) | 87 (12) | ||
| Active asthma [no. (%)] | 0.87 | <0.001 | |||||
| No | 667 (78) | 348 (79) | 319 (76) | 86 (64) | 581 (80) | ||
| Yes | 136 (16) | 72 (16) | 64 (15) | 35 (26) | 101 (14) | ||
| Missing | 54 (6) | 18 (5) | 36 (9) | 13 (10) | 41 (6) | ||
| Prenatal NLEs [mean (± SD)] | 2.5 ± 2.2 | 2.5 ± 2.1 | 2.4 ± 1.9 | 0.57 | 2.9 ± 2.1 | 2.4 ± 2.0 | 0.009 |
| PM2.5 Exposure [ μg/m3 ; mean (± SD)] | 11.0 ± 1.0 | 10.9 ± 0.9 | 11.1 ± 1.0 | 0.10 | 11.1 ± 0.9 | 10.9 ± 1.1 | 0.13 |
| Prenatal Smoking [no. (%)] | 0.69 | 0.09 | |||||
| No | 703 (82) | 362 (83) | 341 (81) | 100 (75) | 603 (83) | ||
| Yes | 125 (15) | 62 (14) | 63 (15) | 26 (19) | 99 (14) | ||
| Missing | 29 (3) | 14 (3) | 15 (4) | 8 (6) | 21 (3) | ||
Abbreviations: Body mass index (BMI), interpersonal trauma (IPT)-categories are mutually exclusive, negative live events (NLEs), particulate matter (PM); Differences in means and proportions were tested using Kruskal-Wallis ANOVA and χ2 test, respectively.
Lifetime Maternal IPT and Child’s Asthma: Logistic Regression
Associations between maternal IPT over the lifecourse and risk of asthma in offspring are presented in Table 2. Relative to women never exposed to IPT, chronic maternal IPT was significantly associated with asthma in the unadjusted model (OR=1.95; 95% CI, 1.18–3.23). After adjusting for maternal age, education, race/ethnicity, and child’s sex, this association remained significant (OR=1.82; 95% CI, 1.06–3.13). In adjusted models stratified by child’s sex, the association between chronic maternal IPT and offspring asthma risk was seen in boys (OR=2.87; 95% CI, 1.48–5.57) but not girls (OR=0.69; 95% CI 0.23–2.12). The interaction between chronic IPT and child’s sex was significant in the adjusted model (pinteraction=0.042).
Table 2.
Associations between maternal lifetime IPT and children’s asthma: Logistic regression models in the total sample and stratified by child’s sex
| Total Sample | ||||
| Model 1a | Model 2b | |||
| OR | 95% CI | OR | 95% CI | |
| No IPT Exposure | Reference | Reference | Reference | Reference |
| Early Life IPT | 1.26 | 0.81–1.96 | 1.24 | 0.78–1.96 |
| Late/Current IPT | 0.77 | 0.32–1.86 | 0.78 | 0.32–1.92 |
| Chronic IPT | 1.95 | 1.18–3.23 | 1.82 | 1.06–3.13 |
| Sex-stratified | ||||
| Model 3 (Boys)c | Model 4 (Girls)c | |||
| No IPT Exposure | Reference | Reference | Reference | Reference |
| Early Life IPT | 1.58 | 0.86–2.90 | 0.85 | 0.41–1.77 |
| Late/Current IPT | 0.73 | 0.21–2.60 | 0.85 | 0.24–3.02 |
| Chronic IPT | 2.87 | 1.48–5.57 | 0.69 | 0.23–2.12 |
Unadjusted;
Adjusted for maternal age, education, race/ethnicity, and child’s sex;
Sex-stratified results adjusted for maternal age, education, race/ethnicity. Abbreviations: interpersonal trauma (IPT)-categories are mutually exclusive. Logistic regression was used to examine the relationship between maternal IPT exposure and child asthma risk.
Path Analyses
Similar to the logistic regression models, effects for chronic IPT were most significantly related to offspring asthma; thus, we present associations between chronic IPT in mothers compared to those reporting no IPT over their lifetime in the path analyses (Figure 1). While the direct effect of maternal chronic IPT was not significant when mediators were included in the model, the path analyses identified significant indirect effects operating through maternal active asthma in the overall sample (Figure 1a). After adjusting for maternal age, education, race/ethnicity, child’s sex, and child’s birthweight z-score, maternal chronic IPT was associated with increased likelihood of maternal active asthma prenatally (β=0.59, p<0.001), and, in turn, children born to mothers with active asthma in pregnancy were more likely to be diagnosed with asthma (β=0.20, p=0.009). The total indirect effect for this path was also significant (β=0.12, p=0.031). Although maternal chronic IPT was positively associated with smoking and the increase of other negative life events experienced during pregnancy, neither were significantly associated with children’s asthma. The total indirect effect of chronic IPT operating through prenatal negative life events was suggestive albeit not significant (β=0.07, p=0.10). Pre-pregnancy BMI and exposure to PM2.5 were significantly associated with asthma in offspring; however, indirect effects of chronic IPT through BMI and PM2.5 were not significant (see Table S1, Supplemental Digital Content, which includes all standardized path coefficients for the path models examining the relationship between maternal chronic IPT and asthma in offspring for the total population and by sex).
Figure 1. Pathways linking maternal chronic IPT to children’s asthma at age 6 years in the total sample (a) and among b) boys and (c) girls.
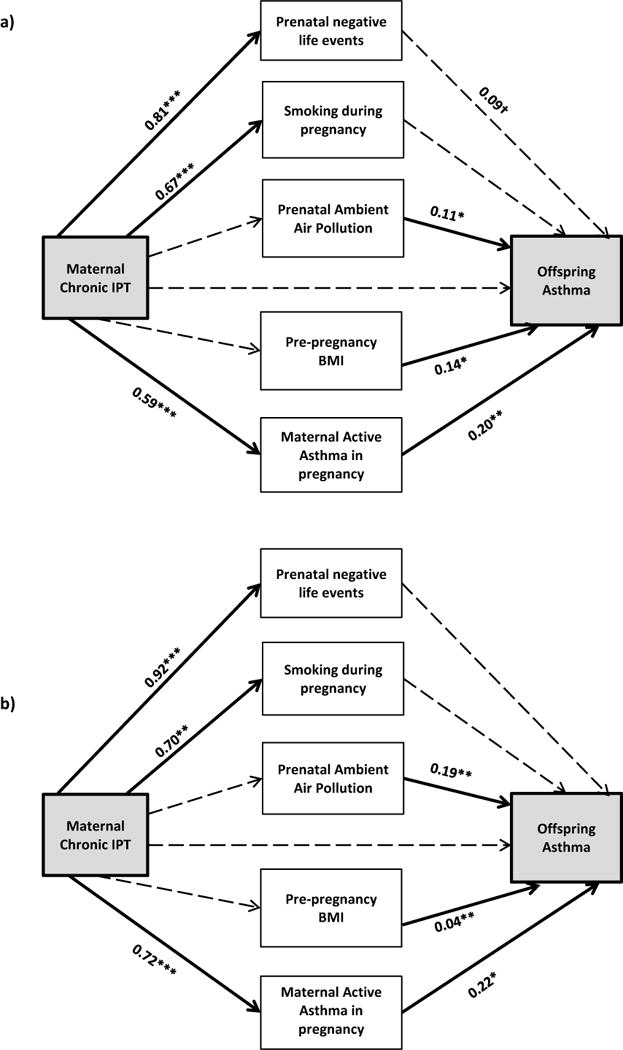
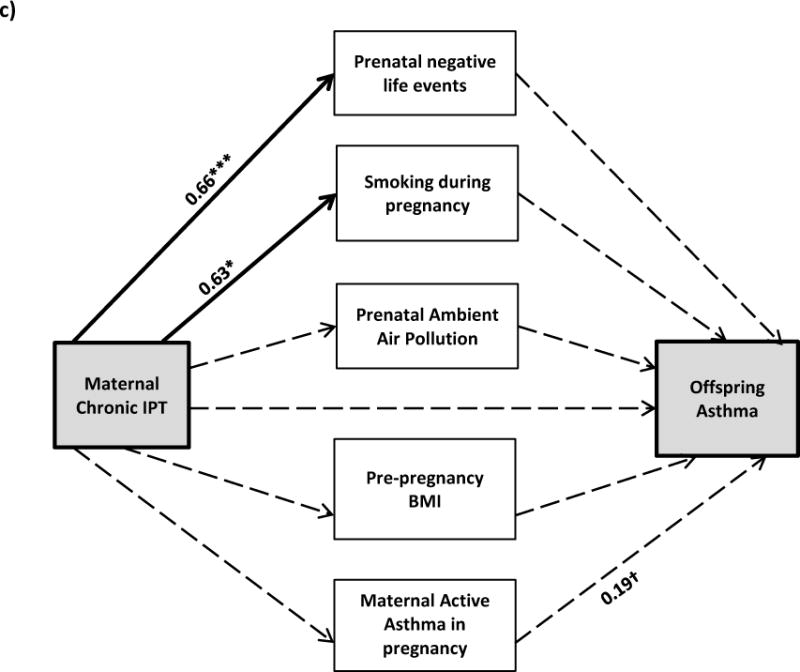
All models are adjusted for maternal race/ethnicity, age, education, and birthweight z-score; model (a) is also adjusted for child’s sex. Bolded lines represent significant pathways; dotted lines represent non-significant pathways (coefficients are listed for significant pathways). †0.05 < p < 0.20, *p < 0.05, ** p < 0.01, ***p < 0.001
Given the significant interaction found in the logistic regression model between child’s sex and maternal chronic IPT, sex-stratified path analyses were conducted. Figure 1 parts b and c summarize the significant path coefficients between chronic maternal IPT and asthma in offspring among boys and girls, respectively. In general, associations between maternal chronic IPT and path variables as well as associations between the path variables and child asthma were stronger in boys compared to girls. Among boys, the relationship between maternal chronic IPT and increased risk for maternal active asthma was significant as was the relationship between maternal active asthma and child’s asthma status; the indirect effect of chronic IPT operating through maternal active asthma did not quite reach statistical significance, likely due to the smaller sample size (β=0.16, p=0.07). Neither the individual effects (chronic IPT predicting maternal active asthma or maternal active asthma predicting child asthma status) nor the total indirect effects (chronic IPT operating through maternal active asthma to predict child asthma status) were significant in girls. As in the overall model, maternal chronic IPT was positively associated with smoking during pregnancy and increased prenatal negative life events in mothers of both boys and girls, although neither was significantly associated with child asthma. Associations between increased PM2.5 and pre-pregnancy BMI on child’s asthma were only observed among boys. Effect estimates for all tested pathways are provided in the online supplement (Table S1).
DISCUSSION
These analyses are the first to demonstrate an association between maternal chronic IPT over the lifecourse and increased asthma risk in children, particularly among boys. Our findings also support some of our hypothesized indirect pathways but not others. Specifically, we show that women experiencing chronic lifetime IPT were more likely to have active asthma during pregnancy which, in turn, was associated with asthma in the next generation. Another suggested pathway was that women experiencing chronic IPT were also more likely to report higher numbers of other negative life events at the time of pregnancy which then, in part, explained the relationship with increased asthma risk in children. However, the latter did not quite reach statistical significance. Path analyses did not support a role for maternal pre-pregnancy obesity, an increased likelihood of maternal smoking during pregnancy, and/or increased exposure to air pollution during pregnancy in mediating the relationship between maternal IPT and childhood asthma.
Maternal asthma history is a well-known risk factor for childhood asthma with prior studies linking active asthma in pregnancy, in particular, to asthma in the next generation.(32, 33) No prior studies have considered chronic stress as an upstream factor that may increase the likelihood that asthma in women may be persistent and active in her childbearing years and during pregnancy. A woman’s exposure to chronic stressors could generate lasting biological changes in her immune and neuroendocrine systems that continue to exert their effects into adulthood and pregnancy. For instance, recent studies document the importance of considering effects of trauma in women on hypothalamic-pituitary-adrenal (HPA) axis functioning in pregnancy (51)given the central role of maternal cortisol production, a hormonal end product of the HPA axis, for healthy fetal development. Schreier et al. recently reported an association between a history of childhood abuse in women with differences in cortisol production during pregnancy(22); others have observed similar results with respect to salivary cortisol.(52) Disrupted maternal cortisol production prenatally has, in turn, been linked to early asthma phenotypes in children.(14) Since chronic stress and increased cortisol can induce a shift in the T-helper (Th) cell 1 balance of peripheral blood mononuclear cells towards a predominantly Th2 response(17) or promote other innate immune processes (e.g., Th17 pathways) that play a role in asthma/allergy,(18) an alteration in maternal cortisol leading up to and persisting during pregnancy could be one mechanism through which chronic IPT contributes to both maternal active asthma and asthma development in the next generation. Chronic traumatic stress has been associated with markers of immune disruption and chronic inflammation in women.(25, 26) Women experiencing abuse and neglect in childhood/adolescence (25) or intimate partner violence (26) exhibited elevated levels of C-reactive protein (CRP), a marker of systemic inflammation, in mid-life compared to women who were not exposed. Lapin and colleagues (27) recently reported an association between elevated levels of CRP in utero and increased likelihood of asthma in children by age 3 years. These data suggest that maternal chronic IPT may be programming fetal immune development and allergic/respiratory outcomes as a result of shifts in the maternal endocrine and immune milieu and pro-inflammatory pathways operating in utero.
Our findings also demonstrate that boys were more affected by maternal chronic IPT exposure in utero. This finding is consistent with animal research showing that while maternal stress in pregnancy affects both sexes, prenatal stress affects males disproportionately.(53) Human studies examining the sex-specific effects of maternal stress on childhood asthma are scarce and contradictory. Using hospital contact data (N > 400,000), Fang et al. (54) observed a faster time to first asthma event prior to age 4 and having a documented asthma attack between the ages of 7–12 years in boys but not girls exposed to prenatal maternal bereavement (a proxy for maternal prenatal stress). A study examining the effect of the 1998 Quebec Ice Storm on pregnant women (prenatal disaster-related stress) showed an increase in physician-diagnosed asthma in the next generation of girls but not boys,(55) although this study was limited by a small sample size and did not adjust for important confounders. More studies examining maternal stress effects on children’s asthma development are needed which include consideration of cumulative traumatic stressors over a mother’s lifecourse with particular attention to sex-specific differences.
Although the exact mechanism for the observed sex differences is unclear, glucocorticoid exposure and metabolism are likely involved. Prenatal glucocorticoid exposure, such as that to naturally occurring cortisol, can lead to oxidative stress and decreased antioxidant defenses, two potential mechanisms implicated in asthma development.(56) Interestingly, the human placenta has been shown to adapt in a sexually dimorphic manner to glucocorticoid exposure. Female placentas are hypersensitive to glucocorticoids in the presence of high levels of cortisol (34) suggesting that female placentas are better able to inactivate cortisol protecting females from excess cortisol and oxidant imbalance. In contrast, the placentas of male fetuses appear to be glucocorticoid resistant in a high cortisol environment making males more vulnerable to an induced pro-oxidant state. (34, 57) This finding might be due, in part, to the presence of several glucocorticoid receptor protein isoforms present in the placenta and the fact that their expression is influenced by not only fetal sex but also maternal asthma, the significant mediator observed in this study. (34) It is important to keep in mind that due to the anti-inflammatory properties of glucocorticoids, they are very effective in the treatment of asthma and thus the first line of defense for asthmatic patients; however, exposure has many side effects and risks. Thus, in addition to the sex-specific effects of maternal stress, likely due to changes in placental function and/or fetal sex-hormone responses (58), the effect of maternal active asthma during pregnancy on placental function may also be attributed to placental glucocorticoid side effects (i.e. oxidative imbalance).(59) Further, evidence from both in vitro and human studies (60–62) show sex-specific global changes in placental gene expression in pregnancies complicated by asthma implicating growth, inflammatory, and immune pathways as additional mechanisms that may explain differential risk for asthma in male and female offspring. Still other studies show sex-specific effects of inhaled corticosteroid (ICS) use in pregnant asthmatics on mechanisms operating at the level of the placenta. (59, 63) Our study did not have a large enough subset of women taking ICSs to look at this specifically. Future studies with larger sample sizes and inclusion of relevant biomarkers will be needed to further elucidate mechanisms underlying these sex-specific effects.
Strengths of this study include the examination of these associations using a lifecourse perspective in a relatively large ethnically-mixed, lower income population with available data on important confounders as well as potential mediating variables. Some limitations should also be noted. Our focus on a sample at higher risk for trauma and asthma may limit generalizability of our findings. Mothers’ history of IPT was self-reported. Notably, women were not aware of the hypotheses being tested when answering the IPT survey and standardized measures as used here which query discrete objective events (e.g., punched, kicked, choked) rather than global perceptions (e.g., were you abused as a child) have good reliability when asked proximate to pregnancy.(64) Moreover, women more typically underreport such events which would drive findings toward the null. While we found a significant relationship between maternal IPT and increased smoking in these pregnant women and that prenatal smoking was more prevalent among women who go on to have a child develop asthma (Table 1), we did not find that smoking in pregnancy mediated the relationship between IPT and children’s asthma. This may be an issue of power and the fact that smoking was a dichotomous indicator (yes/no). Future studies with a larger sample size and the ability to account for level of exposure (i.e., number of cigarettes smoked per day over gestation) may enhance the ability to test this pathway. Lastly, maternal asthma and children’s doctor-diagnosed asthma were reported by mothers. As documented elsewhere (45), the majority of these children were given a diagnosis of asthma after the age of 3 years (78.6%) which reduces the likelihood that cases represented wheezing respiratory illnesses other than asthma (e.g., early transient wheeze), although this remains a possibility. As we follow these children, it will be informative to see if similar associations hold for more objective measures (e.g., spirometry, airway reactivity). Notably, our definition of maternal asthma not only includes the presence of asthma symptoms but also healthcare utilization and the use of asthma medications. Inclusion of these parameters would be expected to minimize under- or over-reporting as studies have shown that self-report agrees with asthma medication possession for most adult asthma patients.(65)
In summary, our results highlight the importance of a lifecourse perspective in examining associations between maternal stress on prenatal programming of children’s asthma rather than solely focusing on stress experienced proximate to the pregnancy. We need to broaden our view to consider stress experienced over the mother’s lifecourse and not just the stress she experiences more immediately around or during pregnancy. Even a woman’s remote exposures to traumatic stressors such as violence and abuse may have particular influence on stress-related programming of respiratory disease in the next generation. Moreover, this relationship appears to be stronger in boys and partially mediated through maternal active asthma in pregnancy, a potential mechanism of intergenerational transmission from parent to offspring. Asthma is one of the most common diseases complicating pregnancy and its prevalence among pregnant women is increasing.(66) The complex nature of the developmental processes that can be affected by the prenatal asthma milieu, suggests increased risk for asthma among offspring is not simply a result of genetic predisposition but likely includes in utero transfer of environmental risk. Moreover, active asthma in pregnancy has been linked to a number of adverse outcomes for the developing child, in addition to asthma development.(33) Thus, these findings have important implications for future studies of the intergenerational effects of stress, pregnancy, and child health more broadly.
Supplementary Material
Acronyms.
IPT, interpersonal trauma; SEM, structural equation modeling; HPA, hypothalamic-pituitary-adrenal; ANS, autonomic nervous system; ACCESS, Asthma Coalition on Community Environment and Social Stress; R-CTS, Revised Conflict Tactics Scale; BMI, body mass index; CRISYS, Crisis in Family Systems-Revised; NLEs, negative life events; PM, particulate matter; MODIS, Moderate Resolution Imaging Spectroradiometer; AOD, Aerosol Optical Depth; LUR, land-use regression; ANOVA, analysis of variance; Th, T-helper; CRP, C-reactive protein; SES, socioeconomic status.
Acknowledgments
Source of Funding: The Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project has been funded by grants R01 ES010932, U01 HL072494, and R01 HL080674 (Wright RJ, PI); phenotyping and biostatistical support was funded by P30 ES023515 and P30 ES000002. During preparation of this manuscript KJB was supported by K99 ES024116 and AL was supported by a Thrasher Research Fund Early Career Award (Lee A, PI), a Chest Foundation Clinical Research Grant (Lee A, PI) and an Empire Clinical Research Investigator Program Award; MJR was supported by T32 HD049311-09.
Footnotes
Conflicts of Interest The authors declare they have no conflicts of interests.
References
- 1.van de Loo KFE, van Gelder MMHJ, Roukema J, Roelveld N, Merkus PJM, Verhaak CM. Prenatal maternal psychological stress and childhood asthma and wheezing - a meta-analysis. Eur Respir J. 2016;47(1):133–46. doi: 10.1183/13993003.00299-2015. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S, Herbert TB. Health psychology: psychological factors and physical disease from the perspective of human psychoneuroimmunology. Annu Rev Psychol. 1996;47:113–42. doi: 10.1146/annurev.psych.47.1.113. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298(14):1685–7. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 4.Cacioppo JT, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Sheridan JF, Poehlmann KM, Burleson MH, Ernst JM, Hawkley LC, Glaser R. Autonomic, neuroendocrine, and immune responses to psychological stress: The reactivity hypothesis. Ann Ny Acad Sci. 1998;840:664–73. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- 5.McEwen BS. Introduction - Protective and damaging effects of stress mediators: The good and bad sides of the response to stress. Metabolism. 2002;51(6):2–4. doi: 10.1053/meta.2002.33183. [DOI] [PubMed] [Google Scholar]
- 6.Licht CMM, Vreeburg SA, Dortland AKBV, Giltay EJ, Hoogendijk WJG, DeRijk RH, Vogelzangs N, Zitman FG, de Geus EJC, Penninx BWJH. Increased Sympathetic and Decreased Parasympathetic Activity Rather Than Changes in Hypothalamic-Pituitary-Adrenal Axis Activity Is Associated with Metabolic Abnormalities. J Clin Endocr Metab. 2010;95(5):2458–66. doi: 10.1210/jc.2009-2801. [DOI] [PubMed] [Google Scholar]
- 7.Yun AJ, Bazar KA, Lee PY. Autonomic dysfunction may be an under-recognized cause of female fertility disorders. Med Hypotheses. 2004;63(1):172–7. doi: 10.1016/j.mehy.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Jankowska EA, Ponikowski P, Piepoli MF, Banasiak W, Anker SD, Poole-Wilson PA. Autonomic imbalance and immune activation in chronic heart failure - Pathophysiological links. Cardiovasc Res. 2006;70(3):434–45. doi: 10.1016/j.cardiores.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 9.de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy - a review. Neurosci Biobehav Rev. 2005;29(2):295–312. doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Arck PC, Knackstedt MK, Blois SM. Current insights and future perspectives on neuroendocrine-immune circuitry challenging pregnancy maintenance and fetal health. J Reprod Endokrinol. 2006;3:98–102. [Google Scholar]
- 11.Yehuda R, Bierer LM. Transgenerational transmission of cortisol and PTSD risk. Prog Brain Res. 2008;167:121–35. doi: 10.1016/S0079-6123(07)67009-5. [DOI] [PubMed] [Google Scholar]
- 12.Lim R, Fedulov AV, Kobzik L. Maternal stress during pregnancy increases neonatal allergy susceptibility: role of glucocorticoids. Am J Physiol Lung Cell Mol Physiol. 2014;307(2):L141–8. doi: 10.1152/ajplung.00250.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Hertzen LC. Maternal stress and T-cell differentiation of the developing immune system: possible implications for the development of asthma and atopy. J Allergy Clin Immunol. 2002;109(6):923–8. doi: 10.1067/mai.2002.124776. [DOI] [PubMed] [Google Scholar]
- 14.Wright RJ, Fisher K, Chiu Y-HM, Wright RO, Fein R, Cohen S, Coull BA. Disrupted prenatal maternal cortisol, maternal obesity, and childhood wheeze: Insights into prenatal programming. Am J Respir Crit Care Med. 2013;187:1186–93. doi: 10.1164/rccm.201208-1530OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beijers R, Jansen J, Riksen-Walraven M, de Weerth C. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics. 2010;126:e401–e9. doi: 10.1542/peds.2009-3226. [DOI] [PubMed] [Google Scholar]
- 16.Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, Lee-Parritz A, Wood RA, Kattan M, Bloomberg GR, Burger M, Togias A, Witter FR, Sperling RS, Sadovsky Y, Gern JE. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. 2010;182(1):25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elenkov IJ, Chrousos GP. Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol Metab. 1999;10(9):359–68. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 18.Carlsson E, Frostell A, Ludvigsson J, Faresjo M. Psychological Stress in Children May Alter the Immune Response. J Immunol. 2014;192(5):2071–81. doi: 10.4049/jimmunol.1301713. [DOI] [PubMed] [Google Scholar]
- 19.Chiu Y-HM, Coull BA, Cohen S, Wooley A, Wright RJ. Prenatal and postnatal maternal stress and wheeze in urban children: effect of maternal sensitization. Am J Respir Crit Care Med. 2012;186:147–54. doi: 10.1164/rccm.201201-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartwig IR, Sly PD, Schmidt LA, van Lieshout RJ, Bienenstock J, Holt PG, Arck PC. Prenatal adverse life events increase the risk for atopic diseases in children, which is enhanced in the absence of a maternal atopic predisposition. J Allergy Clin Immunol. 2014;134(1):160–9. doi: 10.1016/j.jaci.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Cookson H, Granell R, Joinson C, Ben-Shlomo Y, Henderson AJ. Mother’s anxiety during pregnancy is associated with asthma in their children. J Allergy Clin Immunol. 2009;123:847–53. doi: 10.1016/j.jaci.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreier HM, Enlow MB, Ritz T, Gennings C, Wright RJ. Childhood abuse is associated with increased hair cortisol levels among urban pregnant women. J Epidemiol Community Health. 2015 doi: 10.1136/jech-2015-205541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schreier HM, Bosquet Enlow M, Ritz T, Coull BA, Gennings C, Wright RO, Wright RJ. Lifetime exposure to traumatic and other stressful life events and hair cortisol in a multi-racial/ethnic sample of pregnant women. Stress. 2015:1–8. doi: 10.3109/10253890.2015.1117447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corrigan FM, Fisher JJ, Nutt DJ. Autonomic dysregulation and the Window of Tolerance model of the effects of complex emotional trauma. J Psychopharmacol. 2011;25(1):17–25. doi: 10.1177/0269881109354930. [DOI] [PubMed] [Google Scholar]
- 25.Matthews KA, Chang YF, Thurston RC, Bromberger JT. Child abuse is related to inflammation in mid-life women: role of obesity. Brain Behav Immun. 2014;36:29–34. doi: 10.1016/j.bbi.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newton TL, Fernandez-Botran R, Miller JJ, Lorenz DJ, Burns VE, Fleming KN. Markers of inflammation in midlife women with intimate partner violence histories. J Womens Health (Larchmt) 2011;20(12):1871–80. doi: 10.1089/jwh.2011.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lapin B, Ownby D, Turyk M, Piorkowski J, Freels S, Chavez N, Wagner-Cassanova C, Hernandez E, Pelzel D, Vergara C, Persky V. Relationship between in utero C-reactive protein levels and asthma in at-risk children. Ann Allergy Asthma Immunol. 2015;115(4):282–7. doi: 10.1016/j.anai.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Pervanidou P. Biology of post-traumatic stress disorder in childhood and adolescence. J Neuroendocrinol. 2008;20(5):632–8. doi: 10.1111/j.1365-2826.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- 29.Myers HF, Wyatt GE, Ullman JB, Loeb TB, Chin D, Prause N, Zhang M, Williams JK, Slavich GM, Liu H. Cumulative burden of lifetime adversities: Trauma and mental health in low-SES African Americans and Latino/as. Psychol Trauma. 2015;7(3):243–51. doi: 10.1037/a0039077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian SV, Ackerson LK, Subramanyam MA, Wright RJ. Domestic violence is associated with adult and childhood asthma prevalence in India. Int J Epidemiol. 2007;36:569–79. doi: 10.1093/ije/dym007. [DOI] [PubMed] [Google Scholar]
- 31.Romans S, Belaise C, Martin J, Morris E, Raffi A. Childhood abuse and later medical disorders in women. An epidemiological study. Psychother Psychosom. 2002;71(3):141–50. doi: 10.1159/000056281. [DOI] [PubMed] [Google Scholar]
- 32.Martel MJ, Rey E, Beauchesne MF, Malo JL, Perreault S, Forget A, Blais L. Control and severity of asthma during pregnancy are associated with asthma incidence in offspring: two-stage case-control study. Eur Respir J. 2009;34(3):579–87. doi: 10.1183/09031936.00074608. [DOI] [PubMed] [Google Scholar]
- 33.Tegethoff M, Olsen J, Schaffner E, Meinlschmidt G. Asthma during pregnancy and clinical outcomes in offspring: a national cohort study. Pediatrics. 2013;132(3):483–91. doi: 10.1542/peds.2012-3686. [DOI] [PubMed] [Google Scholar]
- 34.Saif Z, Hodyl NA, Hobbs E, Tuck AR, Butler MS, Osei-Kumah A, Clifton VL. The human placenta expresses multiple glucocorticoid receptor isoforms that are altered by fetal sex, growth restriction and maternal asthma. Placenta. 2014;35(4):260–8. doi: 10.1016/j.placenta.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Diesel JC, Bodnar LM, Day NL, Larkby CA. Childhood maltreatment and the risk of pre-pregnancy obesity and excessive gestational weight gain. Matern Child Nutr. 2014 doi: 10.1111/mcn.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng D, Salimi S, Terplan M, Chisolm MS. Intimate partner violence and maternal cigarette smoking before and during pregnancy. Obstet Gynecol. 2015;125(2):356–62. doi: 10.1097/AOG.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wark PAB, Murphy V, Mattes J. The interaction between mother and fetus and the development of allergic asthma. Expert Rev Resp Med. 2014;8(1):57–66. doi: 10.1586/17476348.2014.848795. [DOI] [PubMed] [Google Scholar]
- 38.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CR. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 39.Hien D, Bukszpan C. Interpersonal violence in a “normal” low-income control group. Women Health. 1999;29(4):1–16. doi: 10.1300/J013v29n04_01. [DOI] [PubMed] [Google Scholar]
- 40.Briggs-Gowan MJ, Ford JD, Fraleigh L, McCarthy K, Carter AS. Prevalence of exposure to potentially traumatic events in a healthy birth cohort of very young children in the northeastern United States. J Trauma Stress. 2010;23(6):725–33. doi: 10.1002/jts.20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sternthal MJ, Enlow MB, Cohen S, Canner MJ, Staudenmayer J, Tsang K, Wright RJ. Maternal interpersonal trauma and cord blood IgE levels in an inner-city cohort: a life-course perspective. J Allergy Clin Immunol. 2009;124(5):954–60. doi: 10.1016/j.jaci.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kristiansson M, Sorman K, Tekwe C, Calderon-Garciduenas L. Urban air pollution, poverty, violence and health–Neurological and immunological aspects as mediating factors. Environ Res. 2015;140:511–3. doi: 10.1016/j.envres.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28(36):9055–65. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright RJ, Suglia SF, Levy J, Fortun K, Shields A, Subramanian S, Wright R. Transdisciplinary research strategies for understanding socially patterned disease: the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project as a case study. Cien Saude Colet. 2008;13(6):1729–42. doi: 10.1590/s1413-81232008000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu HL, Chiu YM, Coull BA, Kloog I, Schwartz J, Lee A, Wright RO, Wright RJ. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children: Identifying Sensitive Windows and Sex Differences. Am J Respir Crit Care Med. 2015;192(9):1052–9. doi: 10.1164/rccm.201504-0658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Straus MA, Douglas EM. A short form of the Revised Conflict Tactics Scales, and typologies for severity and mutuality. Violence Vict. 2004;19(5):507–20. doi: 10.1891/vivi.19.5.507.63686. [DOI] [PubMed] [Google Scholar]
- 47.Shalowitz MU, Berry CA, Rasinski KA, Dannhausen-Brun CA. A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Serv Res. 1998;33(5 Pt 1):1381–402. [PMC free article] [PubMed] [Google Scholar]
- 48.Berry CA, Quinn KA, Portillo N, Shalowitz MU. Reliability and validity of the Spanish version of the Crisis in Family Systems-Revised. Psychol Rep. 2006;98:123–32. doi: 10.2466/pr0.98.1.123-132. [DOI] [PubMed] [Google Scholar]
- 49.Kloog I, Koutrakis P, Coull BA, Lee HJ, Schwartz J. Assessing temporally and spatially resolved PM2.5 exposures for epidemiological studies using satellite aerosol optical depth measurements. Atmos Environ. 2011;45(35):6267–75. [Google Scholar]
- 50.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC pediatrics. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bublitz MH, Parade S, Stroud LR. The effects of childhood sexual abuse on cortisol trajectories in pregnancy are moderated by current family functioning. Biol Psychol. 2014;103:152–7. doi: 10.1016/j.biopsycho.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bublitz MH, Stroud LR. Maternal history of child sexual abuse moderates the association between daily stress and diurnal cortisol in pregnancy. Eur J Psychotraumato. 2012;3 doi: 10.3109/10253890.2013.825768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glover V, Hill J. Sex differences in the programming effects of prenatal stress on psychopathology and stress responses: an evolutionary perspective. Physiol Behav. 2012;106(5):736–40. doi: 10.1016/j.physbeh.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Fang F, Hoglund CO, Arck P, Lundholm C, Langstrom N, Lichtenstein P, Lekander M, Almqvist C. Maternal bereavement and childhood asthma-analyses in two large samples of Swedish children. PloS one. 2011;6(11):e27202. doi: 10.1371/journal.pone.0027202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turcotte-Tremblay AM, Lim R, Laplante DP, Kobzik L, Brunet A, King S. Prenatal maternal stress predicts childhood asthma in girls: project ice storm. BioMed Res Int. 2014;2014:201717. doi: 10.1155/2014/201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holguin F. Oxidative stress in airway diseases. Ann Am Thorac Soc. 2013;10(Suppl):S150–7. doi: 10.1513/AnnalsATS.201305-116AW. [DOI] [PubMed] [Google Scholar]
- 57.Stark MJ, Hodyl NA, Wright IM, Clifton VL. Influence of sex and glucocorticoid exposure on preterm placental pro-oxidant-antioxidant balance. Placenta. 2011;32(11):865–70. doi: 10.1016/j.placenta.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Howerton AR, Roland AV, Fluharty JM, Marshall A, Chen A, Daniels D, Beck SG, Bale TL. Sex differences in corticotropin-releasing factor receptor-1 action within the dorsal raphe nucleus in stress responsivity. Biol Psychiatry. 2014;75(11):873–83. doi: 10.1016/j.biopsych.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clifton VL. Sexually dimorphic effects of maternal asthma during pregnancy on placental glucocorticoid metabolism and fetal growth. Cell Tissue Res. 2005;322(1):63–71. doi: 10.1007/s00441-005-1117-5. [DOI] [PubMed] [Google Scholar]
- 60.Osei-Kumah A, Smith R, Jurisica I, Caniggia I, Clifton VL. Sex-specific differences in placental global gene expression in pregnancies complicated by asthma. Placenta. 2011;32(8):570–8. doi: 10.1016/j.placenta.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Scott NM, Hodyl NA, Osei-Kumah A, Stark MJ, Smith R, Clifton VL. The presence of maternal asthma during pregnancy suppresses the placental pro-inflammatory response to an immune challenge in vitro. Placenta. 2011;32(6):454–61. doi: 10.1016/j.placenta.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 62.Murphy VE, Gibson PG, Giles WB, Zakar T, Smith R, Bisits AM, Kessell CG, Clifton VL. Maternal asthma is associated with reduced female fetal growth. Am J Crit Care Med. 2003;168(11):1317–23. doi: 10.1164/rccm.200303-374OC. [DOI] [PubMed] [Google Scholar]
- 63.Clifton V. Maternal asthma during pregnancy and fetal outcomes: potential mechanisms and possible solutions. Curr Opin Allergy Clin Immunol. 2006;6(5):307–11. doi: 10.1097/01.all.0000244788.28789.dd. [DOI] [PubMed] [Google Scholar]
- 64.Cammack AL, Hogue CJ, Drews-Botsch CD, Kramer MR, Pearce BD, TK B, Stowe ZN, Newport DJ. Test-retest reliability of retrospective self-reported maternal exposure to childhood abuse and neglect. Arch Womens Ment Health. 2015 doi: 10.1007/s00737-015-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim KG, Rank MA, Li JT, Patel A, Volcheck GW, Branda ME, Cabanela R, Naessens JM, Shah ND, Wagie A, Beebe T. How well does patient self-report predict asthma medication possession? Implications for medication reconciliation and adherence assessment. J Asthma. 2010;47(8):878–82. doi: 10.3109/02770903.2010.491143. [DOI] [PubMed] [Google Scholar]
- 66.Murphy VE, Schatz M. Asthma in pregnancy: a hit for two. Eur Respir Rev. 2014;23(131):64–8. doi: 10.1183/09059180.00008313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


