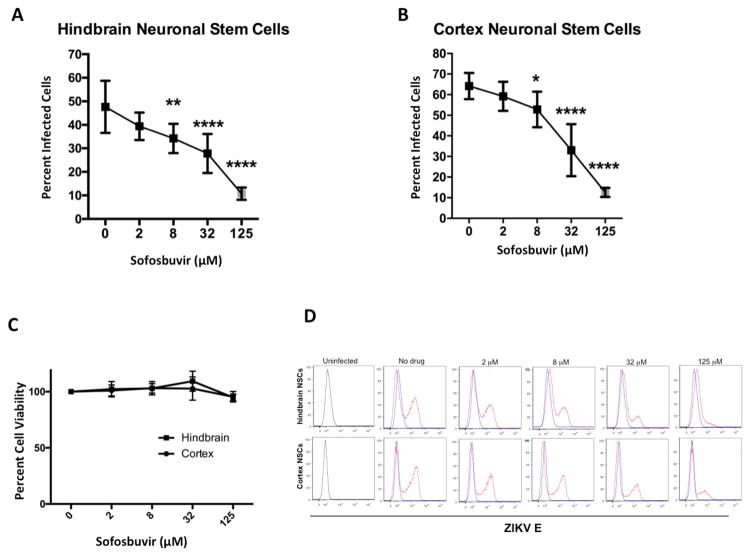
Figure 3. Sofosbuvir reduces ZIKV infection in human fetal-derived neuronal stem cells.
Human NSCs derived from fetal hindbrain or cerebral cortex were infected with ZIKV Paraiba at an MOI of 5 and treated with the indicated concentrations of sofosbuvir. A–B. Forty-eight hours later cells were harvested, fixed, permeabilized, stained with an anti-E protein antibody, and processed by flow cytometry. The data is pooled from three independent experiments, each performed in triplicate. The error bars indicate standard deviations. *, P < 0.05; **, P < 0.01; ****, P < 0.0001 (one-way ANOVA with multiple comparisons correction compared to 0 μM). C. Cytotoxicity analysis of sofosbuvir in human NSCs. NSCs were incubated with the indicated concentrations sofosbuvir for 48 hours, then cell viability was determined using CellTiter-Glo. The data is pooled from two independent experiments performed in duplicate. D. Flow cytometry histograms from data in panels A and B showing inhibitory effect of sofosbuvir against ZIKV infection in human NSCs. One representative experiment of three is shown.