Abstract
PURPOSE
To describe the utility of using wide-angle digital imaging in the training for retinopathy of prematurity (ROP) with laser, and in identifying common locations of skip areas that were present after initial panretinal photocoagulation with indirect ophthalmoscopy by ophthalmologists-in-training.
METHODS
Retrospective review of digital retinal images of 22 eyes of 12 infants who had undergone laser treatment for ROP performed by ophthalmologists-in-training. Presence of skip areas was determined by masked review of photographs. The location of skip areas was classified based on two axes: 1) circumferential (in one of six clock-hour regions), and 2) radial (adjacent to the retinal ridge, adjacent to the ora serrata, or isolated patches of greater than 1 laser burn width).
RESULTS
A total of 30 skip areas were identified in the 22 eyes treated with laser photocoagulation. Based on circumferential location, a significant difference in skip area distribution was found (p=0.02). Regions with the highest percentage of skip areas were between the clock-hours 11:00 to 1:00 (45%), and 5:00 to 7:00 (41%). Based on radial location, 40% of all skip areas were found near the ora serrata, 17% near the ridge, and 43% as isolated patches (P=0.14).
CONCLUSION
Skip areas after indirect panretinal laser photocoagulation by ophthalmologists-in-training were easily visualized by wide-angle digital imaging, after being missed by the trainee during the initial treatment procedure. Most skip areas in this study occurred in the superior or inferior retina. Digital imaging can assist ophthalmologists in visualizing all regions of the retina, can identify inadequate areas of laser treatment, and may reduce the need for re-treatment after initial laser for ROP.
Keywords: Digital imaging, education, panretinal laser photocoagulation, retinopathy of prematurity, skip area
Introduction
Retinopathy of prematurity (ROP) is a vasoproliferative disease of the developing retina that continues to be a leading cause of blindness in the pediatric population.1-3 Treatment criteria for ROP have been validated through the Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) and Early Treatment for Retinopathy of Prematurity (ETROP) trials.4-8 Although anti-VEGF therapy with intravitreal bevacizumab recently has been shown to be effective in promoting regression of ROP,9,10 indirect panretinal laser photocoagulation of the avascular retina continues to remain in our armamentarium for treatment-requiring ROP, preventing its complications of retinal detachment and blindness. 7,8
In indirect panretinal laser photocoagulation treatment of ROP, certain areas of avascular retina may be difficult to identify and thus inadvertently left untreated.11 Sometimes, the full extent of the retina can be technically challenging to visualize with an indirect ophthalmoscope, thus preventing adequate treatment. Previous studies have demonstrated that any avascular skip areas not receiving laser treatment may continue to stimulate VEGF production, resulting in disease progression.11-19 It has also been shown that near-confluent laser treatment of the peripheral avascular retina significantly reduces the rate of disease progression in comparison to treatment with laser spots placed 1 to 1.5 burn widths apart.15 As demonstrated in various studies15,20-24, near-confluent panretinal photocoagulation is the desired treatment endpoint in the laser treatment of ROP.
Indirect panretinal laser photocoagulation requires proficiency with indirect ophthalmoscopy, which may be difficult for the ophthalmologists-in-training. Outcomes of laser photocoagulation in the treatment of ROP have been variable.9, 25,26 Inadequate training may lead to insufficient treatment, which can lead to an increase in ROP recurrence and worse outcomes. Although it has been shown that there is variability in ROP diagnosis by vitreoretinal and pediatric ophthalmology fellows,27,28 performance in ROP treatment and documentation of areas of inadequate treatment, or skip areas, in indirect panretinal laser photocoagulation performed by ophthalmologists-in-training have not been previously studied. This is an important gap in knowledge especially in the training of those who are required to manage ROP. In this study, we aimed to 1) describe the utility of using wide-angle digital imaging in the education of ophthalmologists-in-training (e.g. residents and fellows) learning to treat ROP with laser, and 2) identify, using wide-angle digital imaging, common locations of skip areas that were present after initial panretinal laser photocoagulation with indirect ophthalmoscopy by ophthalmologistsin-training.
Methods
This study was approved by the Weill Cornell Medical College Institutional Review Board.
Study Design
This was a retrospective chart review using digital imaging to identify common locations of skip areas in laser photocoagulation-treated ROP eyes over a 30-month period. During this time span, four ophthalmologists-in-training (vitreoretinal fellows within the first two years of completing their residency training) conducted laser treatment under the direct supervision of an experienced pediatric retina specialist (RVPC).
Routine ROP screening was performed by a pediatric retinal specialist (RVPC) along with ophthalmologists-in-training. Patients underwent laser photocoagulation within 24 hours of diagnosis of treatment-requiring ROP. All patients were treated under general anesthesia, and scleral depression was performed during the laser treatment. Indirect panretinal laser photocoagulation was initially performed by an ophthalmologist-in-training using an 810 nm diode laser. The laser beam, using on an indirect ophthalmoscope, was aimed through the pupil and focused on the peripheral avascular retina using a 20-diopter or 28-diopter condensing lens. Confluent laser treatment was administered with laser burns no more than one burn width apart.
As a standard of care at our institution, in addition to examination performed with indirect ophthalmoscopy by the experienced pediatric retina specialist, immediately after indirect panretinal laser photocoagulation by the ophthalmologist-in-training, wide-angle retinal images were captured using a commercially available camera (RetCam-II, Clarity Medical Systems, Pleasanton, CA). To visualize the entire retina, images were taken in a systematic approach so that all clock hours were imaged adequately. At least 6 images per eye were recorded. Digital images (using live video and static images) were immediately reviewed by the pediatric retina specialist together with the ophthalmologist-in-training to identify and document any skip areas, which were defined as areas of avascular retina 1.5 laser burn width or more in diameter. Any skip areas identified were then lasered by the pediatric retinal specialist (RVPC).
All infants were examined within 1 week of treatment and followed closely until regression of ROP with appropriate vascularization of the retina was observed. Any ROP progression/recurrence, retinal detachment, glaucoma, vitreous hemorrhage, and cataracts were noted.
Data from all patients at Weill Cornell Medical College, NewYork-Presbyterian Hospital who underwent indirect panretinal laser photocoagulation for treatment-requiring ROP as defined by the Early Treatment for Retinopathy of Prematurity (ETROP) trial were included in this study. For the purpose of this study, both electronic hospital medical records and digital images associated with each medical record were reviewed. Eligibility criteria include: 1) Indirect panretinal laser photocoagulation performed initially by an ophthalmologist-in-training under the supervision of a pediatric retinal specialist; 2) At least six high quality digital retinal images for each eye were obtained immediately after laser treatment; 3) Follow up results for each patient were available up to the date of the study. Patients who did not have adequate digital retinal imaging coverage of all clock hours or were transferred to an outside provider for follow up care were excluded.
Data Analysis
For the purpose of this study, photographs taken during laser to determine the need for retreatment were masked and reviewed by a pediatric retinal specialist (RVPC) to identify the presence of skip areas. The location of skip areas was classified based on two axes: 1) circumferential (in one of the following six clock-hour regions: 11:00 to 1:00, 1:00 to 3:00, 3:00 to 5:00, 5:00 to 7:00, 7:00 to 9:00, 9:00 to 11:00), and 2) radial (adjacent to the ridge, adjacent to the ora serrata, or isolated patches of greater than 1.5 laser burn width). Total numbers of skip areas at each location were calculated. Data were analyzed using Pearson's chi-square test with the null hypothesis being no difference in the distribution of skip areas amongst the various clock-hour intervals.
Results
Patient characteristics
Twenty-two eyes of 12 infants who had undergone primary laser treatment for ROP were included for analysis. Mean birth weight of subjects included in this study was 757 grams (range 510 to 945 grams). Mean gestational age at birth was 25 3/7 weeks (range 23 3/7 weeks to 27 5/7 weeks). And mean gestational age at laser was 34 4/7 weeks (range 31 6/7 weeks to 38 1/7 weeks). Of the 22 eyes treated, 12 eyes had zone 2 stage 3 disease with plus disease, and 10 eyes had zone I stage 3 disease with or without plus disease. Neovascularization of the iris was found in 7 of the 22 total eyes prior to surgery. Table 1 shows baseline patient characteristics. All eyes received indirect laser photocoagulation of the avascular retina as the initial treatment. All skip areas identified by digital imaging and indirect ophthalmoscopy by the attending physician were subsequently lasered during the initial laser session. None of the patients included in the study required repeat laser for skip areas on follow up visits.”
Table 1.
Baseline and surgical characteristics of patients.
| Baseline Characteristics | All Patient (N=12) |
|---|---|
| Mean±SD | |
| Gestational Age (weeks) | 25.4±1.5 |
| Birth Weight (grams) | 757.5±97.6 |
| Postmenstrual Age at Laser (weeks) | 34.5±2.2 |
| Length of Follow Up (months) | |
| N (%) | |
| Male Sex (percent) | 7 (58%) |
| Left Eye (percent) | 11 (50%) |
| Disease Classification of Each Eye | N (%) |
| Zone 1/ Stage 3 with or without Plus Disease | 10 (45%) |
| Zone 2/ Stage 3 with Plus Disease | 12 (55%) |
Identification of skip areas based on circumferential location
A total of 30 skip areas were identified in 22 eyes immediately after laser therapy for treatment-requiring ROP by the ophthalmologist-in-training. In 13 of the 22 eyes (59% of eyes), at least one skip area was identified. The performance of the four ophthalmologists-in-training included in the study were similar with 50%, 57%, 60%, and 66% of eyes treated were found to have at least one skip area. Figure 1 shows representative digital retinal images of skip areas identified. Table 2 shows percentage of eyes found with skip areas in each circumferential location. Regions with the highest percentage of skip areas were between the clock-hours 11:00 to 1:00 (45%), followed by 5:00 to 7:00 (41%). The intervals with the most adequate laser treatment and least number of skip areas were 1:00 to 3:00 (5%) and 7:00 to 9:00 clock-hours (9%). A significant difference in skip area distribution was found based on circumferential location (p = 0.02). In addition, for each ophthalmologist-in-training included in the study, chi-square statistic showed no significant deviation from the overall skip area distribution (p = 0.24 to 0.29).
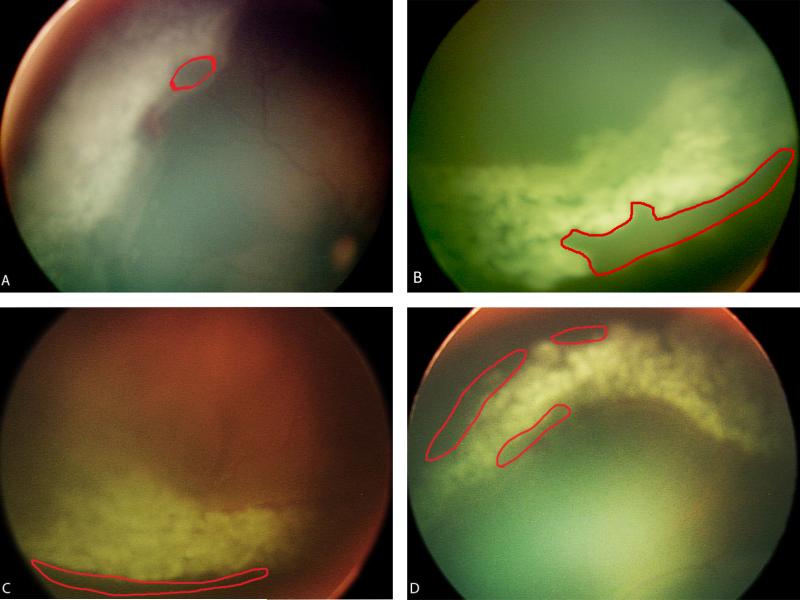
FIGURE 1.
A. Digital retinal image of skip area (demarcated in red) adjacent to the ridge found in the 9:00 to 11:00 clock-hour region. B. Digital retinal image of skip area adjacent to the ora serrata found in the 3:00 to 5:00 clock-hour region. C. Digital retinal image of skip area adjacent to the ridge found in the 5:00 to 7:00 clock-hour region. D. Digital retinal image of several skip areas found in the 11:00 to 1:00 clock-hour region.
Table 2.
Distribution of skip areas based on circumferential location.
| Clock Hour | Number of Skip Areas | Percent of Eyes with Skip Areas | ||
|---|---|---|---|---|
| OS | OD | Overall | ||
| 11 to 1 | 4 | 6 | 10 | 45% |
| 1 to 3 | 0 | 1 | 1 | 5% |
| 3 to 5 | 4 | 1 | 5 | 23% |
| 5 to 7 | 4 | 5 | 9 | 41% |
| 7 to 9 | 1 | 1 | 2 | 9% |
| 9 to 11 | 2 | 1 | 3 | 14% |
Indirect panretinal laser photocoagulation was initially performed by ophthalmologists-in-training in eyes with treatment-requiring ROP. Based on circumferential location, a significant difference in overall skip area distribution was found using Pearson's chi-square test (p=0.02).
Identification of skip areas based on radial location
Table 3 illustrates the distribution of the skip areas based on radial location. Based on radial location, 40% of all skip areas were found anteriorly near the ora serrata, 17% were located posteriorly near the ridge, and 43% of the skip areas were small patches of more than 1.5 burn width apart in between the two skip areas. There was no statistically significant difference when the distribution was classified by radial location (P = 0.14). The majority of skip areas were found in zone II as illustrated in Table 4. Of note, there was no significant difference in the distribution of radial locations of skip areas in zone I treated eyes in comparison to zone II treated eyes. In Table 5, skip areas are classified based on both its circumferential and radial locations in the retina. The number of skip areas found in each specific location of the retina is summarized.
Table 3.
Distribution of the skip areas based on radial location.
| Location of Skip Areas | Total Number of Skip Areas | Percent of All Skip Areas |
|---|---|---|
| Ora Serrata | 12 | 40% |
| Ridge | 5 | 17% |
| Patch | 13 | 43% |
Indirect panretinal laser photocoagulation was initially performed by ophthalmologists-in-training in eyes with treatment-requiring ROP. Based on radial location, no statistically significant difference in the overall distribution of skip areas was found using Pearson's chi-square test (P=0.14).
Table 4.
Distribution of the skip areas based on zone
| Number of Skip Areas | |
|---|---|
| Zone I | 2 |
| Zone II | 25 |
| Zone III | 3 |
Indirect panretinal laser photocoagulation was initially performed by ophthalmologists-in-training in eyes with treatment-requiring ROP. Skip Areas were classified based on its location in different zones of the retina.
Table 5.
Distribution of the skip areas based on both circumferential and radial locations.
| Circumferential Location of Skip Areas | Radial Location of Skip Areas | ||
|---|---|---|---|
| Ora Serrata | Ridge | Patch | |
| 11 to 1 | 4 | 2 | 4 |
| 1 to 3 | 1 | 0 | 0 |
| 3 to 5 | 2 | 0 | 3 |
| 5 to 7 | 3 | 3 | 3 |
| 7 to 9 | 1 | 0 | 1 |
| 9 to 11 | 1 | 0 | 2 |
The locations of skip areas are classified based on both circumferential and radial locations of the retina. The number of skip areas found in each specific location of the retina is shown.
Discussion
The key findings of this study are: 1) Skip areas after laser photocoagulation by ophthalmologists-in-training were commonly detected by wide-angle digital imaging, which were not recognized during the initial treatment procedure by the trainee; 2) Skip areas after laser photocoagulation by ophthalmologists-in-training were commonly located in the superior and inferior clock-hour regions of the retina.
This discrepancy in skip area location after laser photocoagulation by the ophthalmologists-in-training may be attributed to technical difficulty in performing the laser procedure and in visualization of the retina. Due to the neonate's positioning in the neonatal intensive care unit (NICU) sedation suite, it may often be more difficult to obtain a view of the superior retina as compared to other regions of the retina. It is also possible that difficulty in visualization due to vitreous haze and hemorrhage, neovascularization of the iris and any corneal clouding or hyphema resulting from the procedure itself can have an additive effect on difficulty in visualization due to neonatal positioning. While it is possible that each ophthalmologist-in-training may have possessed a different level of skill, which could have led to more skip areas in certain locations due to an outlier effect, no significant deviation from the overall percentage of skip areas at each location were found for the four ophthalmologists-in-training. It is also possible that the circumferential skip area locations can be correlated with the dominant hand of the surgeon such that a right-handed surgeon might have a particularly hard time performing laser superotemporally in a left eye. However, a larger sample size is needed to make conclusions regarding the correlation between surgeon handedness and skip area locations.
Although not statistically significant, there was a trend for skip areas to occur in more anterior locations, when compared to posterior regions adjacent to the neovascular ridge (40% of skip areas were located adjacent to the ora serrata, compared to 17% located near the posterior neovascular ridge). Despite the fact that ROP lasers performed under general anesthesia with scleral depression may reduce the difficulty of reaching the anterior-most avascular regions, we did find a significant number of skip areas anteriorly. It is possible that the peripheral anterior location of the retina near the ora serrata can be difficult to visualize with indirect ophthalmoscopy by the inexperienced examiner. On the other hand, prominent choroidal vessels may be misinterpreted as normal retinal vascularization near the ridge in more posterior disease11. Ophthalmologists-in-training may also be more reluctant to laser close to the ridge because of the fear of causing ridge contraction or hemorrhage, thus inadvertently leaving significant areas of avascular retina untreated. It is possible that with a larger sample size, a statistically significant difference in the distribution of skip areas would have been noted in the anterior-posterior axis.
Although it would also be interesting to define skip areas by percentage of the entire retinal surface, we based our criteria by the results of previously published papers and the ETROP study. As the ETROP study design described, laser treatment was to be performed with burns no more than 1 laser burn width apart20. Other studies have demonstrated treatment failure in patients who had laser with spacing between burns of greater than 1.5 laser burn widths apart,20-24. Therefore, we routinely fill in avascular areas of retina greater than 1 to 1.5 laser burn widths apart.
Technological advancements in imaging have made high-quality photography of the retina possible. Wide-angle fundus documentation using digital video and photography allows instant visualization and real-time documentation of intraocular findings.29 As demonstrated in multiple studies, digital imaging has proven helpful in the screening of ROP, as well as in highlighting plus disease, documenting retinal disease prior to laser surgery, and chorioretinal scar growth after surgery.30-39 In the current study, we demonstrate that digital imaging can be useful in documenting and identifying skip areas that can be missed by initial panretinal laser photocoagulation by ophthalmologists-in-training. We found that digital imaging can help the examiner visualize regions of the retina, including the superior retina, inferior retina and the ora serrata, thus identifying skip areas in difficult locations. In our study, all skip areas were successfully identified using digital imaging when the attending physician or ophthalmologist-in-training operated the camera.
All skip areas found were then lasered after review of the digital image. Additionally, none of the patients in our study were found to have skip areas on follow-up examinations, and all infants had complete regression of ROP at a mean follow up of 11 months. Thus we found that digital imaging may reduce the need for the re-treatment of skip areas after initial laser for ROP by the inexperienced ophthalmologist. This has implications clinically given that skip areas may continue to stimulate VEGF production, resulting in disease progression.
This method has implications not only for patient care but also for education of trainees in managing ROP. Indirect ophthalmoscopy and subsequently indirect panretinal laser photocoagulation for ROP may be technically challenging for the inexperienced examiner. In remote communities and in the developing world where we are facing the issue of lack of adequately trained physicians to manage ROP, the physician, whether a resident, fellow, general ophthalmologist, pediatric ophthalmologist or retina specialist, might be able to use a digital camera to check the work that they have just completed. The inexperienced examiner may be uncertain if they have performed adequate and complete laser treatment. Therefore, through digital imaging of the retina after the inexperienced ophthalmologist feels that they have performed complete laser, it may also be possible to instruct these physicians on locations of skip areas through web-based systems and tele-mentoring.
Our study showed that when laser photocoagulation was performed by ophthalmologistsin-training, a significant number of skip areas was discovered using digital imaging (30 skip areas total in 22 eyes). This finding has potentially important implications for the quality of clinical care and the refinement of fellowship training programs. It was previously shown that a formal evaluation of the competency of ophthalmologists-in-training in ROP management is lacking40. A significant difference in the average number of ROP laser procedures performed by pediatric ophthalmology and retinal fellows also exists40. Currently, the minimum number of all panretinal laser photocoagulation procedures required by the ACGME for ophthalmology residency is twenty41. Significant technical differences exist between the administration of laser by indirect ophthalmoscopy and by slit lamp in the adult and pediatric patient populations. Perhaps, there is a need to establish a minimum number of ROP laser procedures required during fellowship training, much like those required in residency programs, in order to promote uniform training standards. As a standard of care at our institution, after the experienced pediatric retina specialist performs an indirect ophthalmoscopic examination of the patient who had laser treatment by the trainee, a digital image and video is taken and reviewed with the trainee to ensure they are visualizing the areas of incomplete laser. It has been previously suggested that an image-based system that allows for simultaneous viewing by both the retinal specialist and the ophthalmologist-in-training can potentially enhance fellowship education in ROP42.
There are certain limitations that should be considered when interpreting the results of the study: 1) The number of eyes included in our study was relatively small (n=22). Only indirect panretinal laser photocoagulations performed at Weill Cornell Medical College, NewYork-Presbyterian Hospital were included to reduce the effect of confounding due to differences in NICU practices at different institutions. However, our result reached statistical significance based on circumferential location. It is possible that with a larger number of eyes, other significant differences in skip area locations would be noted, particularly when evaluating their anterior-posterior locations. 2) Only four ophthalmologists-in-training participated in the treatment of ROP during the time span of this study. Although each ophthalmologist-in-training may have had a different experience during residency in performing indirect panretinal laser photocoagulation, in order to minimize the effect of confounding due to differences in the levels of procedural skill of these ophthalmologists, all four trainees included were within the first two years of completing residency training when participating in the laser procedure. In addition, the four trainees, individually, showed no difference from the general findings in terms of location of skip areas at completion of laser treatment (p = 0.24 to 0.29). We have also previously shown significant variability in ROP management by vitreoretinal and pediatric ophthalmology fellows using a similar number of trainees as this study and as previously reported, it is possible that there are systemic deficiencies in our training for ROP. 27,28,40 Therefore, these results are potentially generalizable to other ophthalmologists within their first two years of residency who are training to perform ROP laser. Regardless, we acknowledge that a study including a greater number of ophthalmologists at varying years of training will improve the generalizability of the study to all trainees.
In conclusion, as a current standard of care at our institution, we demonstrated the utility of digital imaging in identifying skip areas in the laser treatment of ROP by ophthalmologists-in-training, and we characterized the common locations of these skip areas. The ophthalmologist training for ROP care and treating a patient with ROP should pay particular attention to the superior and inferior retina, as skip areas may occur with higher frequencies in these regions. Digital imaging may be useful in helping the ophthalmologist-in-training and the experienced ophthalmologist in identifying these areas immediately after a laser procedure, in order to adequately complete treatment and reduce the need for re-treatment.
Summary Statement.
Skip areas after laser by ophthalmologists-in-training occurred mainly in the superior or inferior retina. Digital imaging can assist ophthalmologists in visualizing regions of the retina, can identify inadequate areas of laser treatment, has implications for patient care, and can be a training tool for ROP laser.
Acknowledgments
Financial support This study was supported by the St. Giles Foundation (RVPC), grant EY19474 from the National Institutes of Health (MFC), Friends of Doernbecher (MFC), and by the Research to Prevent Blindness (RVPC, MFC, AO, KBK). The sponsors of funding organizations had no role in the design or conduct of this research.
Footnotes
This study was conducted at Weill Cornell Medical College, New York, NY. Presented at the 2012 American Academy of Pediatric Ophthalmology and Strabismus Annual Meeting (San Antonio, TX) and the 2012 Association for Research in Vision and Ophthalmology Annual Meeting (Ft. Lauderdale, FL).
conflicts of interest: The authors have no commercial, proprietary, or financial interest in any of the products or companies described in this article. MFC is an unpaid member of the Scientific Advisory Board for Clarity Medical Systems (Pleasanton, CA).
References
- 1.Flynn JT, Bancalari E, Bachynski BN, Buckley EB, Bawol R, Goldberg R, Cassady J, Schiffman J, Feuer W, Gillings D, et al. Retinopathy of prematurity. Diagnosis, severity, and natural history. Ophthalmology. 1987 Jun;94(6):620–9. [PubMed] [Google Scholar]
- 2.Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert C, Fielder A, Gordillo L, et al. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005;115:e518–e525. doi: 10.1542/peds.2004-1180. [DOI] [PubMed] [Google Scholar]
- 4.Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter trial of cryotherapy for retinopathy of prematurity. Snellen visual acuity and structural outcome at 5 1/2 years after randomization. Arch Ophthalmol. 1996 Apr;114(4):417–24. doi: 10.1001/archopht.1996.01100130413008. [DOI] [PubMed] [Google Scholar]
- 5.Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter trial of cryotherapy for retinopathy of prematurity: natural history ROP: ocular outcome at 5(1/2) years in premature infants with birth weights less than 1251 g. Arch Ophthalmol. 2002 May;120(5):595–9. doi: 10.1001/archopht.120.5.595. [DOI] [PubMed] [Google Scholar]
- 6.Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: ophthalmological outcomes at 10 years. Arch Ophthalmol. 2001 Aug;119(8):1110–8. doi: 10.1001/archopht.119.8.1110. [DOI] [PubMed] [Google Scholar]
- 7.Palmer EA, Hardy RJ, Dobson V, Phelps DL, Quinn GE, Summers CG, Krom CP, Tung B. Cryotherapy for Retinopathy of Prematurity Cooperative Group. 15-year outcomes following threshold retinopathy of prematurity: final results from the multicenter trial of cryotherapy for retinopathy of prematurity. Arch Ophthalmol. 2005 Mar;123(3):311–8. doi: 10.1001/archopht.123.3.311. [DOI] [PubMed] [Google Scholar]
- 8.Good WV, Early Treatment for Retinopathy of Prematurity Cooperative Group Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233–48. [PMC free article] [PubMed] [Google Scholar]
- 9.Mintz-Hittner HA, Kennedy KA, Chuang AZ. BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011 Feb 17;364(7):603–15. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moshfeghi DM, Berrocal AM. Retinopathy of Prematurity in the time of bevacizumab: Incorporating the BEAT-ROP results into clinical practice. Ophthalmology. 2011 Jul;118(7):1227–8. doi: 10.1016/j.ophtha.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Shaikh S, Capone A, Jr, Schwartz SD, Gonzales C, Trese MT. ROP Photographic Screening Trial (Photo-ROP) Study Group. Inadvertent skip areas in treatment of zone 1 retinopathy of prematurity. Retina. 2003 Feb;23(1):128–31. doi: 10.1097/00006982-200302000-00033. [DOI] [PubMed] [Google Scholar]
- 12.Hunter DG, et al. Diode laser photocoagulation for threshold retinopathy of prematurity. A randomized study. Ophthalmology. 1993 Feb;100(2):238–44. doi: 10.1016/s0161-6420(93)31664-7. [DOI] [PubMed] [Google Scholar]
- 13.Vinekar A, et al. Evolution of retinal detachment in posterior retinopathy of prematurity: impact on treatment approach. Am J Ophthalmol. 2008 Mar;145(3):548–555. doi: 10.1016/j.ajo.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 14.MacKeen L, Ells A. Dynamic documentation of the evolution of retinopathy of prematurity in video format. J AAPOS. 2008 Aug;12(4):349–51. doi: 10.1016/j.jaapos.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Banach MJ, Ferrone PJ, Trese MT. A comparison of dense versus less dense diode laser photocoagulation patterns for threshold retinopathy of prematurity. Ophthalmology. 2000 Feb;107(2):324–7. doi: 10.1016/s0161-6420(99)00042-1. [DOI] [PubMed] [Google Scholar]
- 16.Young TL, Anthony DC, Pierce E, Foley E, Smith LE. Histopathology and vascular endothelial growth factor in untreated and diode laser-treated retinopathy of prematurity. J AAPOS. 1997 Jun;1(2):105–10. doi: 10.1016/s1091-8531(97)90008-2. [DOI] [PubMed] [Google Scholar]
- 17.Houston SK, Wykoff CC, Berrocal AM, Hess DJ, Murray TG. Laser treatment for retinopathy of prematurity. Lasers Med Sci. 2011 Dec 2; doi: 10.1007/s10103-011-1021-z. [DOI] [PubMed] [Google Scholar]
- 18.Seiberth V, Linderkamp O, Vardarli I, Knorz MC, Liesenhoff H. Diode laser photocoagulation for stage 3+ retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 1995 Aug;233(8):489–93. doi: 10.1007/BF00183430. [DOI] [PubMed] [Google Scholar]
- 19.Tsitsis T, Tasman W, McNamara JA, Brown G, Vander J. Diode laser photocoagulation for retinopathy of prematurity. Trans Am Ophthalmol Soc. 1997;95:231–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy RJ, Good WV, Dobson V, Palmer EA, Phelps DL, Quintos M, Tung B. Early Treatment for Retinopathy of Prematurity Cooperative Group. Multicenter trial of early treatment for retinopathy of prematurity: study design. Control Clin Trials. 2004 Jun;25(3):311–25. doi: 10.1016/j.cct.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Drenser KA, Trese MT, Capone A., Jr Aggressive posterior retinopathy of prematurity. Retina. 2010 Apr;30(4 Suppl):S37–40. doi: 10.1097/IAE.0b013e3181cb6151. [DOI] [PubMed] [Google Scholar]
- 22.Rezai KA, Eliott D, Ferrone PJ, Kim RW. Near confluent laser photocoagulation for the treatment of threshold retinopathy of prematurity. Arch Ophthalmol. 2005 May;123(5):621–6. doi: 10.1001/archopht.123.5.621. [DOI] [PubMed] [Google Scholar]
- 23.Vander JF. Ophthalmology. 2000 Feb;107(2):328. Discussion by James F. Vander, MD. [Google Scholar]
- 24.Gonzalez VH, et al. Confluent laser photocoagulation for the treatment of retinopathy of prematurity. J AAPOS. 2010 Mar-Apr;47(2):81–5. doi: 10.3928/01913913-20100308-05. quiz 86-7. [DOI] [PubMed] [Google Scholar]
- 25.Early Treatment for Retinopathy of Prematurity Cooperative Group. Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Tung B, Redford M. Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol. 2010 Jun;128(6):663–71. doi: 10.1001/archophthalmol.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jalali S, Kesarwani S, Hussain A. Outcomes of a protocol-based management for zone 1 retinopathy of prematurity: the Indian Twin Cities ROP Screening Program report number 2. Am J Ophthalmol. 2011 Apr;151(4):719–724. doi: 10.1016/j.ajo.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Myung JS, Paul Chan RV, Espiritu MJ, Williams SL, Granet DB, Lee TC, Weissgold DJ, Chiang MF. Accuracy of retinopathy of prematurity image-based diagnosis by pediatric ophthalmology fellows: implications for training. J AAPOS. 2011 Dec;15(6):573–8. doi: 10.1016/j.jaapos.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul Chan RV, Williams SL, Yonekawa Y, Weissgold DJ, Lee TC, Chiang MF. Accuracy of retinopathy of prematurity diagnosis by retinal fellows. Retina. 2010 Jun;30(6):958–65. doi: 10.1097/IAE.0b013e3181c9696a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seiberth V, Woldt C. Wide angle fundus documentation in retinopathy of prematurity. Ophthalmologe. 2001 Oct;98(10):960–3. doi: 10.1007/s003470170044. [DOI] [PubMed] [Google Scholar]
- 30.Lorenz B, Spasovska K, Elflein H, Schneider N. Wide-field digital imaging based telemedicine for screening for acute retinopathy of prematurity (ROP): Six-year results of a multicentre field study. Graefes Arch Clin Exp Ophthalmol. Epub. 2009 May 22; doi: 10.1007/s00417-009-1077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Photographic Screening for Retinopathy of Prematurity (Photo-ROP) Cooperative Group The photographic screening for retinopathy of prematurity study (photo-ROP). Primary outcomes. Retina. 2008 Mar;28(3 Suppl):S47–54. doi: 10.1097/IAE.0b013e31815e987f. [DOI] [PubMed] [Google Scholar]
- 32.Salcone EM, Johnston S, VanderVeen D. Review of the use of digital imaging in retinopathy of prematurity screening. Semin Ophthalmol. 2010 Sep-Nov;25(5-6):214–7. doi: 10.3109/08820538.2010.523671. [DOI] [PubMed] [Google Scholar]
- 33.Chiang MF, Wang L, Busuioc M, Du YE, Chan P, Kane SA, Lee TC, Weissgold DJ, Berrocal AM, Coki O, Flynn JT, Starren J. Telemedical retinopathy of prematurity diagnosis: accuracy, reliability, and image quality. Arch Ophthalmol. 2007 Nov;125(11):1531–8. doi: 10.1001/archopht.125.11.1531. [DOI] [PubMed] [Google Scholar]
- 34.Scott KE, Kim DY, Wang L, Kane SA, Coki O, Starren J, Flynn JT, Chiang MF. Telemedical diagnosis of retinopathy of prematurity intraphysician agreement between ophthalmoscopic examination and image-based interpretation. Ophthalmology. 2008 Jul;115(7):1222–1228. e3. doi: 10.1016/j.ophtha.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Richter GM, Williams SL, Starren J, Flynn JT, Chiang MF. Telemedicine for retinopathy of prematurity diagnosis: evaluation and challenges. Surv Ophthalmol. 2009 Nov-Dec;54(6):671–85. doi: 10.1016/j.survophthal.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagawa TA, Skrinska R. Improved documentation of retinal hemorrhages using a wide-field digital ophthalmic camera in patients who experienced abusive head trauma. Arch Pediatr Adolesc Med. 2001 Oct;155(10):1149–52. doi: 10.1001/archpedi.155.10.1149. [DOI] [PubMed] [Google Scholar]
- 37.Cabrera MT, Freedman SF, Kiely AE, Chiang MF, Wallace DK. Combining ROP tool measurements of vascular tortuosity and width to quantify plus disease in retinopathy of prematurity. J AAPOS. 2011 Feb;15(1):40–4. doi: 10.1016/j.jaapos.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Lepore D, Molle F, Pagliara MM, Baldascino A, Angora C, Sammartino M, Quinn GE. Atlas of fluorescein angiographic findings in eyes undergoing laser for retinopathy of prematurity. Ophthalmology. 2011 Jan;118(1):168–75. doi: 10.1016/j.ophtha.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 39.Lee TC, Lee SW, Dinkin MJ, Ober MD, Beaverson KL, Abramson DH. Chorioretinal scar growth after 810-nanometer laser treatment for retinoblastoma. Ophthalmology. 2004 May;111(5):992–6. doi: 10.1016/j.ophtha.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 40.Wong RK, Ventura CV, Espiritu MJ, Yonekawa Y, Henchoz L, Chiang MF, Lee TC, Chan RVP. Training fellows for retinopathy of prematurity care: a web-based survey. JAPPOS. doi: 10.1016/j.jaapos.2011.12.154. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Accreditation Council for Graduate Medical Education [December 27, 2011];Ophthalmology Resident Operative Minimum Requirements. http://www.acgme.org/acWebsite/navPages/nav_240.asp.
- 42.Wallace DK. Fellowship training in retinopathy of prematurity. J AAPOS. 2012 Feb;16(1):1. doi: 10.1016/j.jaapos.2011.11.003. [DOI] [PubMed] [Google Scholar]