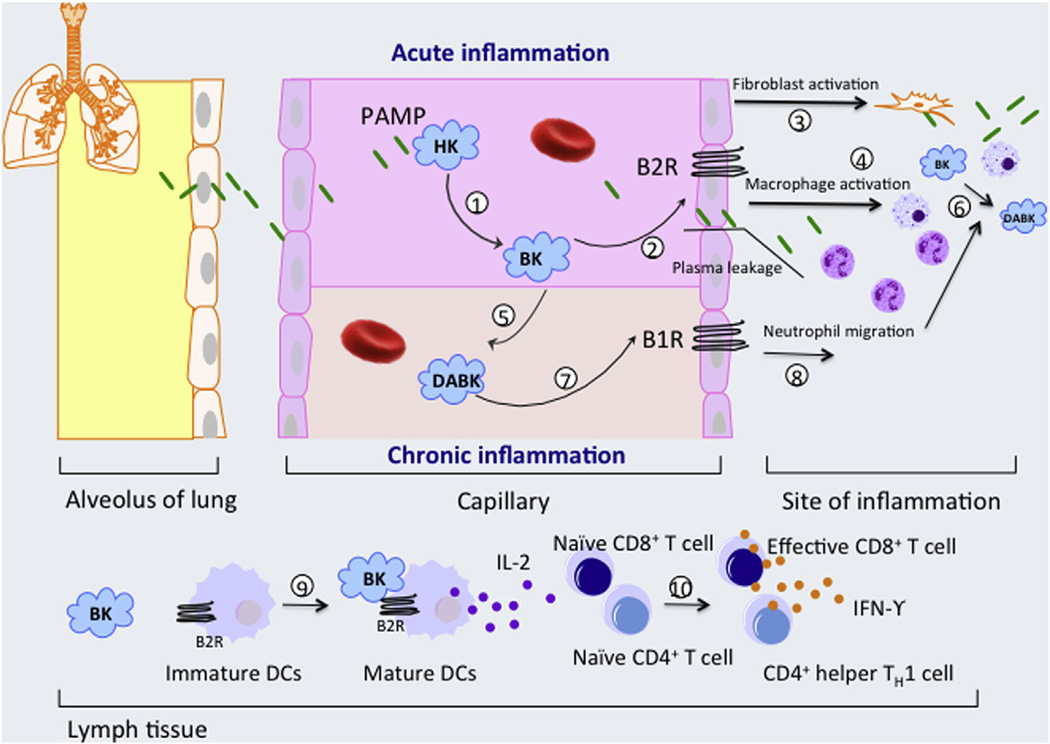
Fig. 1.
Role of pathogen–associated molecular pattern (PAMP) contact activation in inflammation. (1) In circulation, PAMPs interact with high molecular weight kininogen (HK) to activate the contact system and liberate bradykinin (BK) from HK [15]. (2) BK interacts with bradykinin B2 receptors (B2Rs) on vascular endothelial cells to augment plasma extravasation during acute inflammation [23], while also activating lung fibroblasts (3) and macrophages to promote (4) pulmonary neutrophil and monocyte accumulation [18,19,50]. BK is also converted to DABK by CPN in circulation (5) and CPM at sites of inflammation (6), which can act through B1R to regulate permeability and chemokine production properties of endothelial cells as well as enhance neutrophil migration, accumulation and modulate the life span of neutrophils at sites of inflammation (8). BK also induces the migration and maturation of dendritic cell (DC) via the B2R signaling pathways (9). Mature DC producing IL-12 and presenting peptide-MHC complexes, and therefore promote priming and differentiation of naive CD4+ T cells and CD8+ T lymphocytes into CD4+ Th1 and CD8+ cytotoxic effector T cells (10). B1 receptor (B1R) can be induced by BK and other pro-inflammatory cytokines. Also, monocyte stimulation after bacterial toxin release can secrete cytokines which in turn trigger the increase of B1R [51]. The function of DABK via B1R is not completely understood but its up-regulation was found in persistent chronic inflammation [30].