Abstract
Neurocognitive impairment (NCI) and treatment engagement (TE) have been shown to significantly predict antiretroviral therapy (ART) adherence, but no studies have explored the ways and the extent to which similar outcomes might occur when these factors operate together, particularly for people who use drugs (PWUDs). We sought to discover whether TE moderated the effect of NCI on adherence to ART in HIV-infected individuals. 116 HIV-infected, methadone-maintained people who reported HIV risk behaviors were enrolled in the study. Variables of interest (NCI, ART adherence, TE) were assessed using audio computer assisted self-interview. Results revealed a significant interactive effect of NCI and TE on ART adherence, which supported the moderation effect. Findings from post hoc analyses showed that NCI was negatively associated with adherence to ART at low levels of TE. Findings suggest the need to accommodate individual NCI and improve TE as a means to enhance ART adherence in HIV-infected PWUDs.
Keywords: ART adherence, drug users, HIV, methadone maintenance treatment, neurocognitive impairment, treatment engagement
The HIV epidemic continues to be a major global health issue, with approximately 37 million people living with HIV (PLWH) worldwide (Joint United Nations Programme on HIV/AIDS, 2015), and substance use has been closely linked with HIV since the beginning of the epidemic. This link has to do with the increased risk of both contracting and transmitting HIV during drug use and of a worsening of the consequences of HIV infection. People who use drugs (PWUDs) are a critical population for spread of HIV infection, which may occur through behavioral disinhibition, associated with preventable HIV risk behaviors (e.g., inconsistent condom use, sharing of injection equipment; Arasteh, Jarlais, & Perlis, 2008; Marshall et al., 2014; Noar, 2008; Strathdee et al., 2010; Volkow & Montaner, 2011).
Furthermore, increasing evidence exploring the consequences of prolonged drug use on the brain has demonstrated a variety of negative impacts on the central nervous system, thus resulting in neurocognitive impairment (NCI) symptoms. Prior evidence has demonstrated that HIV-infected PWUDs display a wide range of cognitive deficits including problems with executive function, attention, memory, new learning, information-processing speed, and visual-spatial perception, that have significant impact on HIV risk behaviors and risk-reduction intervention outcomes. Furthermore, the presence of cognitive impairment may be associated with the disease process (AIDS-related dementia), drug use history, or relatively poor lifestyle (Anand, Springer, Copenhaver, & Altice, 2010; Anderson, Higgins, Ownby, & Waldrop-Valverde, 2015; Attonito, Devieux, Lerner, Hospital, & Rosenberg, 2014; Becker, Thames, Woo, Castellon, & Hinkin, 2011; Byrd et al., 2011; Ezeabogu, Copenhaver, & Potrepka, 2012; Heaton et al., 2011; Meade, Conn, Skalski, & Safren, 2011; Schouten, Cinque, Gisslen, Reiss, & Portegies, 2011; Shrestha, Weikum, Copenhaver, & Altice, 2016; Thaler, Sayegh, Kim, Castellon, & Hinkin, 2015; Woods, Moore, Weber, & Grant, 2009; Zhou &Saksena, 2013) and may be disruptive to participation in treatment services, including HIV prevention, treatment engagement, and medication adherence, which must be accounted for during behavioral intervention development and adaptation (Bates, Pawlak, Tonigan, & Buckman, 2006; Fishbein et al., 2007; Huedo-Medina, Shrestha, &Copenhaver, 2016; Shrestha & Copenhaver, 2016; Shrestha, Huedo-Medina, & Copenhaver, 2015; Verdejo-Garcia & Perez-Garcia, 2007; Vo, Schacht, Mintzer, & Fishman, 2014).
With recent advances in prophylactic and therapeutic strategies, such as antiretroviral therapy (ART), the life expectancy of PLWH has increased. Thus, improvements in health-related quality of life have become a key focus for researchers and health care providers (Clayson et al., 2006). Adherence to ART by PWUDs is challenging, however, due to distinct concerns faced by drug users, such as regimen complexity, pill burden, side effects, untreated depression, substance use, and lack of social support (Ammassari et al., 2001; Bartlett, 2002; Bartlett, DeMasi, Quinn, Moxham, & Rousseau, 2001; Wagner et al., 2011). PLWH, and particularly PWUDs with HIV infection, are at increased risk of experiencing NCI such that it may significantly impede their abilities to partake fully in treatment services, treatment engagement, and ART adherence (Bates et al., 2006; Fishbein et al., 2007; Shrestha, Huedo-Medina, et al., 2015; Verdejo-Garcia & Perez-Garcia, 2007; Vo et al., 2014).
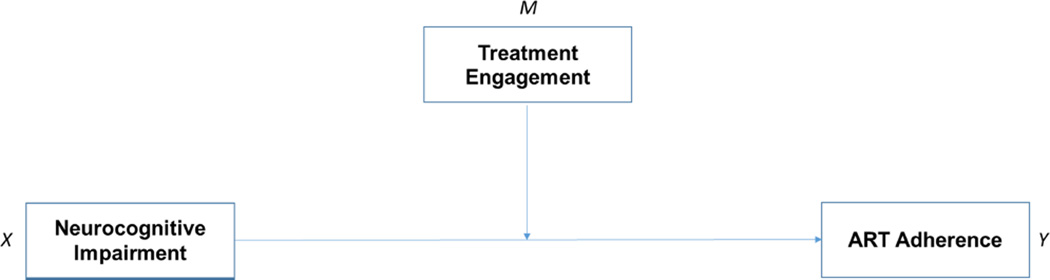
Researchers have recognized NCI and treatment engagement as significant predictors of ART adherence (Farrell, Ingersoll, & Ceperich, 2009; Malee et al., 2009; Meade et al., 2011; Nicholas et al., 2014). Although informative, studies so far have been focused on explaining the independent direct effect of these factors on ART adherence by PLWH. Thus, the possible ways and the extent to which a similar outcome could occur when these factors operate together or interact with each other, particularly for PWUDs, remains an important unanswered question. A better understanding of the extent of the interactive effect (i.e., moderated effect) of NCI and treatment engagement on ART adherence is essential for developing interventions intended to enhance medication adherence in this high-risk population. In our study, we, therefore, sought evidence about the interactive effect of NCI and treatment engagement on ART adherence in HIV-infected PWUDs in treatment. The hypotheses tested in our study included: (a) NCI will be inversely associated with ART adherence; (b) treatment engagement will be positively associated with ART adherence; and (c) treatment engagement will moderate the effect of NCI on ART adherence. Figure 1 shows the conceptual diagram of the moderated model tested in the study.
Figure 1.
The proposed conceptual scheme for moderated model in the study.
Note. ART = antiretroviral therapy.
Methods
We used data from the Holistic Health for HIV (3H+) project collected from September 2012 to December 2015 to examine the relationships between NCI, treatment engagement, and ART adherence in the study population. The 3H+ project was a randomized controlled trial designed to compare the efficacy of the abbreviated 3H+ intervention (Shrestha, Krishnan, Altice, & Copenhaver, 2015) with the Holistic Health Recovery Program (HHRP+), an existing evidence-based behavioral intervention (Margolin, Avants, Warburton, Hawkins, & Shi, 2003) to reduce HIV-related risk behaviors in HIV-infected drug users in New Haven, Connecticut (Shrestha, Krishnan, et al., 2015).
The Institutional Review Board at the University of Connecticut and the Human Investigation Committee at Yale University approved the protocol of this study. Additionally, board approval from the APT Foundation Methadone Maintenance Program, Inc. (the research site) was received. Clinical trial registration was completed at www.ClinicalTrials.gov (NCT01741311).
Participants
This study included 116 (male = 68) HIV-infected, opioid-dependent individuals enrolled in methadone maintenance treatment (MMT) in New Haven, Connecticut. Additional inclusion criteria included: being 18 years of age or older; reporting drug-related (e.g., needle sharing) or sex-related (e.g., inconsistent condom use) HIV risk behavior in the previous 6 months; able to read and understand questionnaires; and not actively suicidal, homicidal, or psychotic.
Measures
Demographic variables
These included participant characteristics including age, gender, ethnicity, education level, marital status, income, methadone dose, and length of drug use history.
Neurocognitive impairment
NCI of the participants was measured using the Brief Inventory of Neurocognitive Impairment (BINI; Copenhaver, Shrestha, Wickersham, Weikum, & Altice, 2016). The BINI is a brief, self-report measure of neuropsychological symptoms, which was developed as a quick and convenient way to help assess diagnostically pertinent information about general and specific cognitive symptoms (e.g., memory, learning, linguistic, academic). The BINI is comprised of 57 items and 9 factors that demonstrate excellent overall reliability (α = 0.97). Factors include: global impairment (e.g., I have difficulty paying attention and I get lost easily); academic-related (e.g., I count with my fingers and I have trouble learning new things); language-related (e.g., My words get mixed up); memory-related (e.g., I have trouble remembering people’s names); psychomotor/physical (e.g., I am very clumsy); psychomotor/perceptual (e.g., I have trouble with the left side of my body); anger-related (e.g., I have urges to break and smash things); pain-associated (e.g., I have severe headaches); and traumatic head injury-related (e.g., I have been knocked unconscious). The reliability of the nine factors ranged from excellent to good (F1 α = 0.97 to F9 α = 0.73). In our study, we used global impairment as a measure of NCI, which included 22 items. The NCI scale was utilized as a continuous variable, with a higher score indicating a greater degree of NCI.
Treatment engagement
As in prior studies, treatment engagement was defined as participants’ rates of attendance during the intervention sessions (Gopalan et al., 2010; Lindsey et al., 2014; Littell, Alexander, & Reynolds, 2001). Treatment engagement was assessed by calculating the number of groups attended by participants during the 12 weeks of the intervention period with a range from 1 to 12. Treatment attendance was used as a continuous variable with higher group attendance indicating greater treatment engagement.
ART adherence
ART adherence was assessed using the empirically validated, self-report visual analog scale (VAS) approach (Giordano, Guzman, Clark, Charlebois, & Bangsberg, 2004). In this method, participants were asked to indicate the percentage of ART medication taken as directed in the previous month by pointing along a continuous line between 0% and 100%. Higher scores indicated a greater degree of ART adherence.
Procedures
Participants enrolled at the APT Foundation, a community-based MMT facility in New Haven, Connecticut, were screened for participation in the study. Those who met the inclusion criteria of the study were invited to provide informed consent, followed by a baseline assessment. Participants were then randomized to receive (a) the Holistic Health for HIV (3H+) intervention: 4 weekly group sessions and the 12-week booster session; or (b) the Holistic Health Recovery Program Plus (HHRP+) intervention: 12 weekly group sessions.
Holistic Health for HIV (3H+)
The 3H+ intervention, which included 4 weekly 60-minute group sessions and a 60-minute booster session at the twelfth week, contained only content that related explicitly to drug- or sex-related HIV risk reduction and ART adherence (Copenhaver, Lee, Margolin, Bruce, & Altice, 2011). 3H+ is a modified coping skills training approach that is delivered in a group setting by two trained intervention facilitators using a motivational enhancement therapeutic style to address high risk drug- and sex- related HIV risk behaviors and ART adherence.
Holistic Health Recovery Program Plus (HHRP+)
The comparison intervention condition – HHRP+ – has been identified by the Centers for Disease Control and Prevention as an evidence-based intervention targeting HIV-infected drug users. It is comprised of 12 weekly group sessions with wide-ranging HIV risk reduction content that addresses the medical, social, emotional, and spiritual needs of PLWH. Each session is designed to last 2 hours and is co-facilitated by two trained facilitators (Margolin et al., 2003).
Participants were assessed at baseline, immediately at post-intervention, 3-month follow-up, 6-month follow-up, and 9-month follow-up using audio computer assisted self-interview (Turner, Rogers, Hendershot, Miller, & Thornberry, 1996). Everyone enrolled in the study was reimbursed a total of $750 for the time and effort required to participate in all the assessments.
Data Analyses
Data analyses were performed using Statistical Analysis Software (SAS, version 9.4) at a 95% confidence interval level (α < .05). We performed Pearson's correlations to identify significant associations for NCI at baseline, treatment engagement during intervention phase, and ART adherence at 9-month follow-up. Outcome data (i.e., ART adherence) was included from the 9-month follow-up because any significant changes that occurred between pre-, post-, and 3- and 6-month follow-ups would tend to weaken the magnitude of influence. This allowed us to examine the influence of NCI on ART adherence over time. We tested our hypotheses in two linked steps. To illustrate, the main effect terms (NCI and treatment engagement) were entered on the first step, and the interaction term (NCI × treatment engagement) was entered on the second step, according to the method for determining moderator effects proposed by Baron and Kenny (1986). All analyses were controlled for gender, income, education status, methadone dose, drug use history, and treatment group. After the moderation test, significant moderators were further analyzed using the post hoc method suggested by Holmbeck (2002). Two additional regressions were run in which the slope between the independent variable (NCI) and the dependent variable (probability of being ART-adherent) was observed when the moderator was re-centered at one standard deviation above and one standard deviation below its mean.
Results
Sample Characteristics
Socio-demographic characteristics of the participants are shown in Table 1. The mean age of the study sample was 48.8 years (SD = 8.5) and more than half (58.6%) were male. Thirty-seven percent of the study participants were African American, followed by Hispanic (31.9%), White (29.3%), and others (1.7%). More than half of the participants (52.6%) had never married. Of the total participants, only 15.5% were high-school graduates, with the majority earning less than $11,000 USD per year. All participants were enrolled in an inner-city MMT program and were maintained on a stable dose. The mean (± SD) daily methadone dose was 77.9 (± 31.56) mg. The participants reported using drugs (e.g., opiate, cocaine) regularly for about 22.72 (± 10.2) years.
Table 1.
Characteristics of the Study Participants (N = 116)
| Variables | Frequency | Percentage |
|---|---|---|
| Age, M (± SD) | 48.8 (± 8.5) | |
| Gender | ||
| Male | 68 | 58.6 |
| Female | 48 | 41.4 |
| Ethnicity | ||
| White | 34 | 29.3 |
| African American | 43 | 37.1 |
| Hispanic | 37 | 31.9 |
| Others | 2 | 1.7 |
| Education level | ||
| Did not finish High School (< 12thgrade) | 98 | 84.5 |
| Finished High School (> 12thgrade) | 18 | 15.5 |
| Marital status | ||
| Married | 14 | 12.1 |
| Never married | 61 | 52.6 |
| Separated | 13 | 11.2 |
| Divorced | 24 | 20.7 |
| Widowed | 4 | 3.4 |
| Income | ||
| 0 – $10,999 | 98 | 84.5 |
| $11,000 – $20,999 | 14 | 12.1 |
| $21,000 – $30,000 | 2 | 1.7 |
| > $30,000 | 2 | 1.7 |
| Methadone dose, M (± SD) | 77.90 (± 31.56) | |
| Length of drug use (in years), M (± SD) | 22.72 (± 10.20) |
Note. SD = standard deviation.
Table 2 presents the summary statistics and correlations of variables of interest for the participants. The score for NCI ranged from 0 to 162, with a mean score of 63.78 (± 36.54). Also, the average total score was 6.94 (± 3.61) for treatment engagement and 91.6 (± 16.10) for ART adherence. An inspection of the correlations revealed that NCI was significantly and negatively related to ART adherence (r = −0.305, p = .016), whereas, treatment engagement was significantly and positively related to ART adherence (r = 0.321, p = .011).
Table 2.
Summary Statistics and Correlations of Variables of Interest in the Participants (N = 116)
| NCI | Treatment engagement |
ART adherence |
M | SD | Range | |
|---|---|---|---|---|---|---|
| NCI | 1 | 63.78 | 36.54 | 0 – 162 | ||
| Treatment engagement | −0.190 | 1 | 6.94 | 3.61 | 1 – 12 | |
| ART adherence | −0.284* | 0.321* | 1 | 91.60 | 16.10 | 0 – 99 |
Note. NCI = neurocognitive impairment; ART = antiretroviral therapy; SD = standard deviation; Higher scores indicate higher levels of neurocognitive impairment, treatment engagement, and ART adherence.
p < 0.05
Test of Moderation
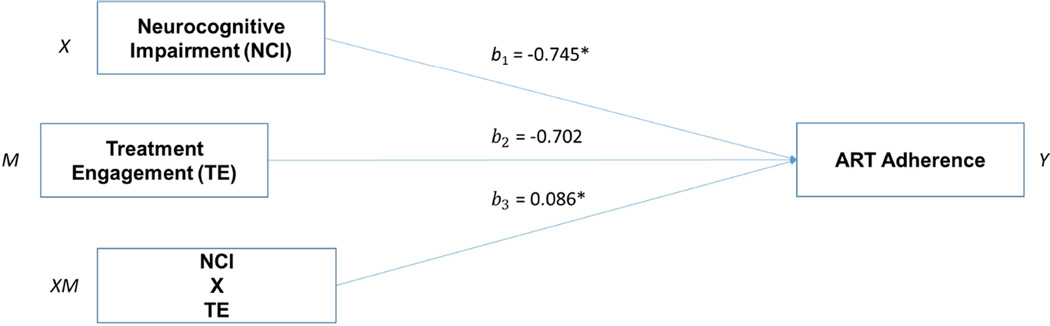
Table 3 presents the results of the regression analyses for moderation. As hypothesized, NCI was negatively associated with ART adherence (B = −.745, p = .004). Treatment engagement was, however, not significantly associated with ART adherence (B = −.702, p = .468). And finally, an interaction term between NCI and treatment engagement was significantly associated with ART adherence (B = .086, p = .023). This significant interactive effect supported our third hypothesis of a moderating effect of treatment engagement on the relationship between NCI and ART adherence (Table 3, Figure 2). The results of the post hoc analyses showed that NCI had significant negative effect on ART adherence at low levels of treatment engagement (Effect = −.4592, p = .0033). This effect was, however, non-significant at greater levels of treatment engagement. Thus, at a low level of treatment engagement, NCI will be associated with lower levels of ART adherence.
Table 3.
Regression Results for Moderation Model (N = 116)
| Variable | B | SE | t | p | CI | |
|---|---|---|---|---|---|---|
| NCI | −.745 | .245 | −3.035 | .004 | −1.236, −0.254 | |
| Treatment Engagement | −.702 | .961 | −0.731 | .468 | −2.625, 1.221 | |
| Interaction terma | .086 | .037 | 2.337 | .023 | 0.012, 0.159 | |
| Conditional effect of NCI on ART Adherence at Values of the Treatment Engagement | ||||||
| Treatment Engagement | Effect | SE | t | p | CI | |
| 3.3282 | −.4592 | .149 | −3.066 | .0033 | −.759, 1.1593 | |
| 6.9355 | −.1495 | .123 | −1.212 | .2305 | −.397, .098 | |
| 10.5427 | .1602 | .208 | .771 | .4437 | −.256, .5759 | |
Note. NCI = neurocognitive impairment; ART = antiretroviral therapy; SE = standard error; B = standardized coefficients; CI = confidence interval; all analyses controlled for gender, income, education status, methadone dose, drug use history, and treatment group.
Interaction term: NCI * Treatment Engagement
Figure 2.
Moderation model of neurocognitive impairment, treatment engagement, and ART adherence.
Note. NCI = neurocognitive impairment; ART = antiretroviral therapy; TE = treatment engagement; all analyses controlled for gender, income, education status, methadone dose, drug use history, and treatment group.
Discussion
To our knowledge, our study is the first to assess the influence of NCI, treatment engagement, and the interactive effect of NCI and treatment engagement on ART adherence within the context of HIV-infected PWUDs. Findings here suggest that the relationship between NCI and ART adherence is more complicated than previous studies have indicated (Farrell et al., 2009; Malee et al., 2009; Meade et al., 2011; Nicholas et al., 2014). Initially, we examined whether NCI would affect ART adherence. We then determined whether treatment engagement would strengthen or weaken the effect of NCI on ART adherence. The results supported the hypothesized moderation model, demonstrating that the magnitude of the effect of NCI on ART adherence was contingent upon an individual’s level of engagement in treatment. This result established the preliminary evidence of a previously unexplored domain (i.e., treatment engagement) influencing the impact of NCI on ART adherence for HIV-infected drug users in treatment.
The findings from our study contribute to the existing literature by first reinforcing and then extending prior results. Our results emphasized the impact of NCI, as it showed the main effect on ART adherence. Consistent with previous findings, our study showed the significant effect of the presence and severity of NCI on decreased ART adherence (Cook et al., 2014; Hinkin et al., 2002; Malee et al., 2009; Waldrop-Valverde, Jones, Weiss, Kumar, & Metsch, 2008). This finding was significant for HIV-infected individuals, particularly PWUDs, who had a greater likelihood of being cognitively impaired, exhibiting depressive symptoms, and reduced social support due to disease process, lifestyles, and chronic substance use behaviors (Bhatia, Hartman, Kallen, Graham, & Giordano, 2011; Scheyett et al., 2010; Zahari et al., 2010). As a result, there was an increased likelihood of suboptimal ART adherence through the complex interaction of NCI, depression, and reduced social support.
Moreover, the findings from our study contribute to research on NCI and how it may interact with treatment engagement to impact ART adherence in this high-risk population. As an extension of prior findings, our results showed a moderating effect of treatment engagement on the association between NCI and ART adherence. That is, treatment engagement had a significant impact of NCI on ART adherence, and particularly so for those with lower treatment engagement reinforcing the negative influence of NCI on adherence. By incorporating a moderated model, we are able to assert that the main effect of NCI on ART adherence works differently in subgroups of individuals. Specifically, HIV-infected PWUDs with lower levels of treatment engagement are likely to experience an increased negative contribution of NCI on ART adherence. This finding highlights the importance of precisely targeting NCI and treatment engagement, while developing interventions to improve ART adherence for HIV-infected PWUDs.
Our data suggest that cognitive impairment and lower treatment engagement may together be significant predictors of ART adherence for HIV-infected PWUDs. Improving ART adherence may depend on first determining whether cognitive functioning is impaired and then making appropriate accommodations. As such, a systematic assessment of cognitive functioning should be used in routine clinical assessment, and interventions should be tailored to address and take into account the possible effect of NCI in this high-risk population. Ignoring NCI issues will likely result in lower rates of ART adherence.
Additionally, our analyses suggest some interesting directions for future research. For example, we did not assess perceived or available social support, depressive symptoms, or substance use disorders, all of which may impact the influence of NCI on ART adherence. Future studies that expand on the proposed model to include such variables may further contribute to this area of inquiry. Furthermore, future studies need to investigate the moderated model in different study samples to confirm its generalizability.
Limitations
The findings from our study need to be considered in light of a few inherent limitations. First, we utilized self-report assessments, which may have been subject to biases associated with participants’ tendencies to misrepresent levels of ART adherence (i.e., over-reporting adherence in VAS scale) and awareness about particular items related to NCI (i.e., underreport NCI on the BINI). This potential bias may have been diminished, however, by the use of audio computer assisted self-interview, which provided participants with a high level of privacy. Second, the BINI, although a very user-friendly and convenient screening instrument for difficult-to-reach populations, was not designed to provide a comprehensive assessment of NCI, and does not measure all possible cognitive domains. Third, the use of the single-item VAS scale to measure participant adherence to ART may not have captured the complex patterns of adherence behavior in HIV-infected individuals. Fourth, our results are specific to HIV-infected drug users in the context of a treatment setting and may not be generalizable to other risk populations in different geographical contexts. Regardless of these limitations, the results of the study have several clinical and research implications and add to our understanding of the processes by which NCI may interact with treatment engagement to affect ART adherence.
Conclusion
Our findings contribute to a burgeoning literature on NCI as a key factor associated with ART adherence in HIV-infected PWUDs (Farrell et al., 2009; Malee et al., 2009; Meade et al., 2011; Nicholas et al., 2014). Furthermore, the results provide preliminary evidence of an interaction effect via NCI and treatment engagement, such that NCI has an increased negative effect on ART adherence for individuals with lower treatment engagement. The results of our study, therefore, make a significant contribution to our understanding of the applicability of a moderated model for improving ART adherence by PLWH with some form of cognitive deficit. Given the greater likelihood of cognitive deficits in this high-risk population (Farrell et al., 2009; Malee et al., 2009; Meade et al., 2011; Nicholas et al., 2014), future interventions seeking to improve ART adherence should include accommodating NCI as a primary goal
Key Considerations.
Neurocognitive impairment (NCI) and treatment engagement (TE) are significant predictors of antiretroviral therapy (ART) adherence.
The results revealed a significant interactive effect of NCI and TE on ART adherence in HIV-infected drug users in treatment.
NCI was significantly negatively associated with adherence to ART at low levels of TE.
Future interventions seeking to improve ART adherence in HIV-infected drug users in treatment should accommodate individuals' NCI and improve TE.
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse for research (R01 DA032290) and for career development (K02 DA033139) to Michael Copenhaver.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no real or perceived vested interests that relate to this article that could be construed as a conflict of interest.
Contributor Information
Roman Shrestha, Department of Community Medicine & Health Care, University of Connecticut Health Center, Farmington, Connecticut, USA (Roman.Shrestha@UConn.edu).
Pramila Karki, Department of Allied Health Sciences, University of Connecticut, Storrs, Connecticut, USA.
Tania B. Huedo-Medina, Department of Allied Health Sciences, University of Connecticut, Storrs, Connecticut, USA.
Michael Copenhaver, Department of Allied Health Sciences, University of Connecticut, Storrs, Connecticut, USA.
References
- Ammassari A, Murri R, Pezzotti P, Trotta MP, Ravasio L, De Longis P, Antinori A. Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2001;28(5):445–449. doi: 10.1097/00042560-200112150-00006. [DOI] [PubMed] [Google Scholar]
- Anand P, Springer SA, Copenhaver MM, Altice FL. Neurocognitive impairment and HIV risk factors: A reciprocal relationship. AIDS and Behavior. 2010;14(6):1213–1226. doi: 10.1007/s10461-010-9684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AM, Higgins MK, Ownby RL, Waldrop-Valverde D. Changes in neurocognition and adherence over six months in HIV-infected individuals with cocaine or heroin dependence. AIDS Care. 2015;27(3):333–337. doi: 10.1080/09540121.2014.985183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasteh K, Jarlais DCD, Perlis TE. Alcohol and HIV sexual risk behaviors among injection drug users. Drug & Alcohol Dependence. 2008;95(1):54–61. doi: 10.1016/j.drugalcdep.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attonito JM, Devieux JG, Lerner BD, Hospital MM, Rosenberg R. Exploring substance use and hiv treatment factors associated with neurocognitive impairment among people living with HIV/AIDS. Frontiers in Public Health. 2014;2:105. doi: 10.3389/fpubh.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bartlett JA. Addressing the challenges of adherence. Journal of Acquired Immune Deficiency Syndromes. 2002;29(Suppl-1):S2–S10. doi: 10.1097/00126334-200202011-00002. [DOI] [PubMed] [Google Scholar]
- Bartlett JA, DeMasi R, Quinn J, Moxham C, Rousseau F. Overview of the effectiveness of triple combination therapy in antiretroviral-naive HIV-1 infected adults. AIDS. 2001;15(11):1369–1377. doi: 10.1097/00002030-200107270-00006. [DOI] [PubMed] [Google Scholar]
- Bates ME, Pawlak AP, Tonigan JS, Buckman JF. Cognitive impairment influences drinking outcome by altering therapeutic mechanisms of change. Psychology of Addictive Behaviors. 2006;20(3):241–253. doi: 10.1037/0893-164X.20.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker BW, Thames AD, Woo E, Castellon SA, Hinkin CH. Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS and Behavior. 2011;15(8):1888–1894. doi: 10.1007/s10461-011-9924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia R, Hartman C, Kallen MA, Graham J, Giordano TP. Persons newly diagnosed with HIV infection are at high risk for depression and poor linkage to care: Results from the Steps Study. AIDS and Behavior. 2011;15(6):1161–1170. doi: 10.1007/s10461-010-9778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DA, Fellows RP, Morgello S, Franklin D, Heaton RK, Deutsch R, Grant I. Neurocognitive impact of substance use in HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2011;58(2):154–162. doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson DJ, Wild DJ, Quarterman P, Duprat-Lomon I, Kubin M, Coons SJ. A comparative review of health-related quality-of-life measures for use in HIV/AIDS clinical trials. Pharmacoeconomics. 2006;24(8):751–765. doi: 10.2165/00019053-200624080-00003. [DOI] [PubMed] [Google Scholar]
- Cook R, Waldrop-Valverde D, Sharma A, Vamos S, Mahajan B, Weiss SM, Jones DL. Cognitive functioning, depression, and HIV medication adherence in India: A randomized pilot trial. Health Psychology and Behavioral Medicine. 2014;2(1):640–652. doi: 10.1080/21642850.2014.913487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhaver M, Shrestha R, Wickersham JA, Weikum D, Altice FL. An exploratory factor analysis of a brief self-report scale to detect neurocognitive impairment among participants enrolled in methadone maintenance therapy. Journal of Substance Abuse Treatment. 2016;63(4):61–65. doi: 10.1016/j.jsat.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhaver MM, Lee IC, Margolin A, Bruce RD, Altice FL. Testing an optimized community-based human immunodeficiency virus (HIV) risk reduction and antiretroviral adherence intervention for HIV-infected injection drug. Substance Abuse. 2011;32(1):16–26. doi: 10.1080/08897077.2011.540466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeabogu I, Copenhaver MM, Potrepka J. The influence of neurocognitive impairment on HIV treatment outcomes among drug-involved people living with HIV/AIDS. AIDS Care. 2012;24(3):386–393. doi: 10.1080/09540121.2011.608794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell L, Ingersoll K, Ceperich SD. Enhancing patient adherence: Promoting engagement via positive patient-provider relationships in HIV/AIDS care. Medical Encounter. 2009;23(2):69–71. [PMC free article] [PubMed] [Google Scholar]
- Fishbein DH, Krupitsky E, Flannery BA, Langevin DJ, Bobashev G, Verbitskaya E, Tsoy M. Neurocognitive characterizations of Russian heroin addicts without a significant history of other drug use. Drug and Alcohol Dependence. 2007;90(1):25–38. doi: 10.1016/j.drugalcdep.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clinical Trials. 2004;5(2):74–79. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- Gopalan G, Goldstein L, Klingenstein K, Sicher C, Blake C, McKay MM. Engaging families into child mental health treatment: Updates and special considerations. Journal of the Canadian Academy of Child & Adolescent Psychiatry. 2010;19(3):182–196. [PMC free article] [PubMed] [Google Scholar]
- Heaton R, Franklin D, Ellis R, McCutchan JA, Letendre S, LeBlanc S, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. Journal of NeuroVirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin C, Castellon S, Durvasula R, Hardy D, Lam M, Mason K, Stefaniak M. Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59(12):1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27(1):87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Huedo-Medina TB, Shrestha R, Copenhaver M. Modeling a theory-based approach to examine the influence of neurocognitive impairment on HIV Risk reduction behaviors among drug users in treatment. AIDS and Behavior. 2016:1–12. doi: 10.1007/s10461-016-1394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS. How AIDS changed everything. 2015 Retrieved from http://www.unaids.org/sites/default/files/media_asset/MDG6Report_en.pdf. [Google Scholar]
- Lindsey MA, Brandt NE, Becker KD, Lee BR, Barth RP, Daleiden EL, Chorpita BF. Identifying the common elements of treatment engagement interventions in children’s mental health services. Clinical Child and Family Psychology Review. 2014;17(3):283–298. doi: 10.1007/s10567-013-0163-x. [DOI] [PubMed] [Google Scholar]
- Littell JH, Alexander LB, Reynolds WW. Client participation: Central and underinvestigated elements of intervention. Social Service Review. 2001;75(1):1–28. [Google Scholar]
- Malee K, Williams PL, Montepiedra G, Nichols S, Sirois PA, Storm D, Kammerer B. The role of cognitive functioning in medication adherence of children and adolescents with HIV infection. Journal of Pediatric Psychology. 2009;34(2):164–175. doi: 10.1093/jpepsy/jsn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin A, Avants SK, Warburton LA, Hawkins KA, Shi J. A randomized clinical trial of a manual-guided risk reduction intervention for HIV-positive injection drug users. Health Psychology. 2003;22(2):223–228. [PubMed] [Google Scholar]
- Marshall BDL, Friedman SR, Monteiro JFG, Paczkowski M, Tempalski B, Pouget ER, Galea S. Prevention and treatment produced large decreases in HIV incidence in a model of people who inject drugs. Health Affairs. 2014;33(3):401–409. doi: 10.1377/hlthaff.2013.0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Conn NA, Skalski LM, Safren SA. Neurocognitive impairment and medication adherence in HIV patients with and without cocaine dependence. Journal Behavioral Medicine. 2011;34(2):128–138. doi: 10.1007/s10865-010-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas PK, Willard S, Thompson C, Dawson-Rose C, Corless IB, Wantland DJ, Moezzi S. Engagement with care, substance use, and adherence to therapy in HIV/AIDS. AIDS Research and Treatment. 2014;2014 doi: 10.1155/2014/675739. Article ID 675739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noar SM. Behavioral interventions to reduce HIV-related sexual risk behavior: Review and synthesis of meta-analytic evidence. AIDS and Behavior. 2008;12(3):335–353. doi: 10.1007/s10461-007-9313-9. [DOI] [PubMed] [Google Scholar]
- Scheyett A, Parker S, Golin C, White B, Davis CP, Wohl D. HIV-infected prison inmates: Depression and implications for release back to communities. AIDS and Behavior. 2010;14(2):300–307. doi: 10.1007/s10461-008-9443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten J, Cinque P, Gisslen M, Reiss P, Portegies P. HIV-1 infection and cognitive impairment in the cART era: A review. AIDS. 2011;25(5):561–575. doi: 10.1097/QAD.0b013e3283437f9a. [DOI] [PubMed] [Google Scholar]
- Shrestha R, Copenhaver M. The influence of neurocognitive impairment on HIV risk behaviors and intervention outcomes among high-risk substance users: A systematic review. Frontiers in Public Health. 2016;4(16) doi: 10.3389/fpubh.2016.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha R, Huedo-Medina TB, Copenhaver MM. Sex-related differences in self-reported neurocognitive impairment among high-risk cocaine users in methadone maintenance treatment program. Substance Abuse: Research and Treatment. 2015;9:17–24. doi: 10.4137/SART.S23332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha R, Krishnan A, Altice FL, Copenhaver M. A non-inferiority trial of an evidence-based secondary HIV prevention behavioral intervention compared to an adapted, abbreviated version: Rationale and intervention description. Contemporary Clinical Trials. 2015;44:95–102. doi: 10.1016/j.cct.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha R, Weikum D, Copenhaver M, Altice FL. The influence of neurocognitive impairment, depression, and alcohol use disorders on health-related quality of life among incarcerated, HIV-infected, opioid dependent Malaysian men: A moderated mediation analysis. AIDS and Behavior. 2016:1–12. doi: 10.1007/s10461-016-1526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Hallett TB, Bobrova N, Rhodes T, Booth R, Abdool R, Hankins CA. HIV and risk environment for injecting drug users: The past, present, and future. Lancet. 2010;376(9737):268–284. doi: 10.1016/S0140-6736(10)60743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler NS, Sayegh P, Kim MS, Castellon SA, Hinkin CH. Interactive effects of neurocognitive impairment and substance use on antiretroviral non-adherence in HIV disease. Archives of Clinical Neuropsychology. 2015;30(2):114–121. doi: 10.1093/arclin/acu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CF, Rogers SM, Hendershot TP, Miller HG, Thornberry JP. Improving representation of linguistic minorities in health surveys. Public Health Reports. 1996;111(3):276–279. [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M. Profile of executive deficits in cocaine and heroin polysubstance users: Common and differential effects on separate executive components. Psychopharmacology. 2007;190(4):517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- Vo HT, Schacht R, Mintzer M, Fishman M. Working memory impairment in cannabis- and opioid-dependent adolescents. Substance Abuse. 2014;35(4):387–390. doi: 10.1080/08897077.2014.954027. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Montaner J. The urgency of providing comprehensive and integrated treatment for substance abusers with HIV. Health Affairs. 2011;30(8):1411–1419. doi: 10.1377/hlthaff.2011.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GJ, Goggin K, Remien RH, Rosen MI, Simoni J, Bangsberg DR, Liu H. A closer look at depression and its relationship to HIV antiretroviral adherence. Annals of Behavioral Medicine. 2011;42(3):352–360. doi: 10.1007/s12160-011-9295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop-Valverde D, Jones DL, Weiss S, Kumar M, Metsch L. The effects of low literacy and cognitive impairment on medication adherence in HIV-positive injecting drug users. AIDS Care. 2008;20(10):1202–1210. doi: 10.1080/09540120801927017. [DOI] [PubMed] [Google Scholar]
- Woods S, Moore D, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychology Review. 2009;19(2):152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahari MM, Bae WH, Zainal NZ, Habil H, Kamarulzaman A, Altice FL. Psychiatric and substance abuse comorbidity among HIV seropositive and HIV seronegative prisoners in Malaysia. American Journal of Drug and Alcohol Abuse. 2010;36(1):31–38. doi: 10.3109/00952990903544828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Saksena NK. HIV associated neurocognitive disorders. Infectious Disease Reports. 2013;5(Suppl. 1):e8. doi: 10.4081/idr.2013.s1.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]