Figure 2.
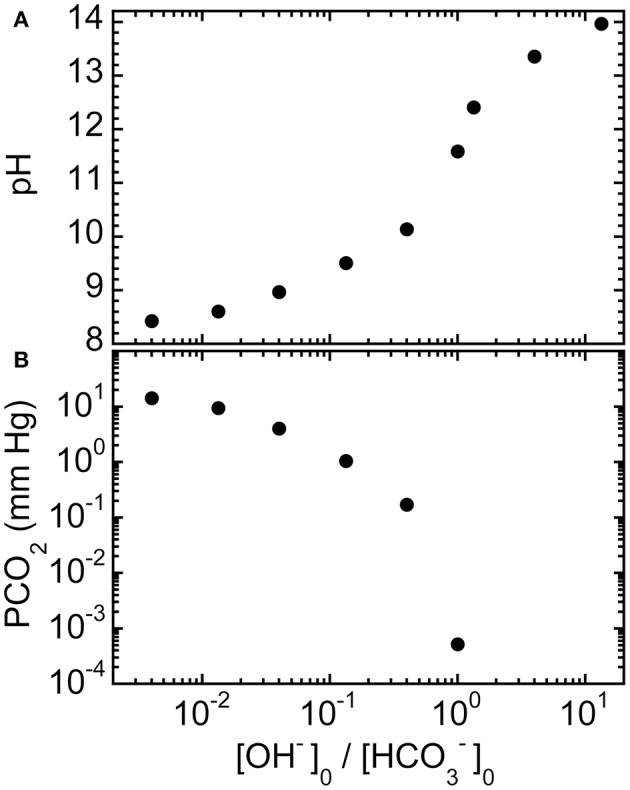
Predicted equilibrium properties of ROMB solutions composed of NaHCO3 and NaOH. (A) pH and (B) PCO2 of ROMB solutions resulting from mixing different proportions of a 150 mM NaOH solution to a 150 mM NaHCO3 solution, expressed by the ratio of the initial concentration of hydroxide ions [OH−]0 divided by the initial concentration of bicarbonate anions []0. The total initial concentration of basic species is fixed at 150 mM. For ratios greater than 1, PCO2 has a vanishingly small value and is not shown.
