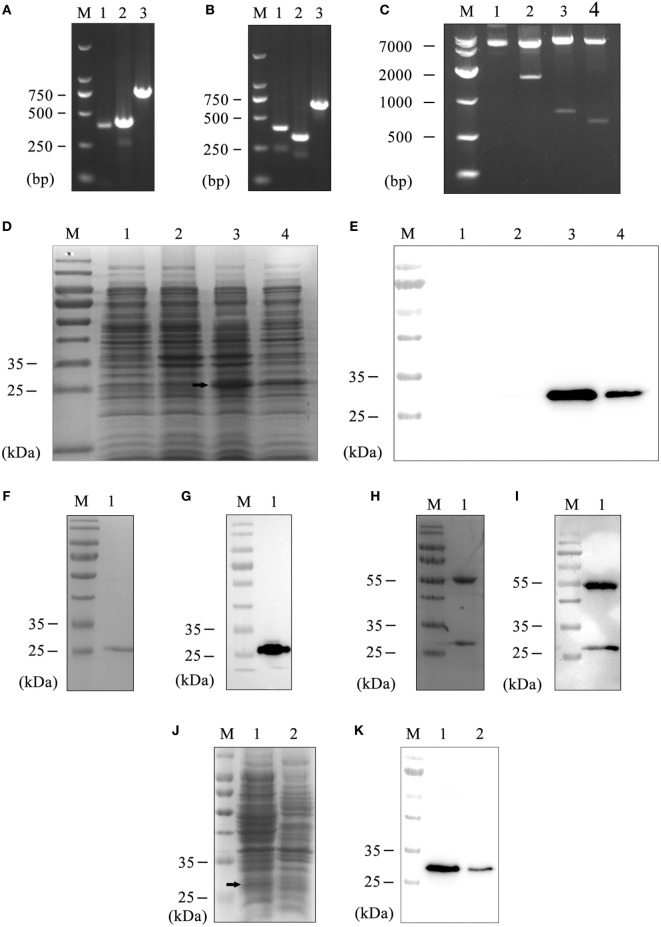
Figure 1.
Preparation of the hS9-Fab03. (A) The heavy chain PCR products of positive clone. Lane 1, VH; Lane 2, CH; Lane 3, VH combined with CH; Lane M, DNA maker. (B) The light chain PCR products of positive clone. Lane 1, VL; Lane 2, CL; Lane 3, VL combined with CL; Lane M, DNA maker. (C) The construction of pETDuet-hSiglec-9 Fab03. Lane 1, the plasmid without restriction endonuclease digestion; Lane 2, the plasmid was double digested with NcoI and XhoI; Lane 3, NcoI and HindIII were used for double digesting the plasmid; Lane 4, EcoRV and XhoI were used for double digesting the plasmid; Lane M, DNA marker. Coomassie blue staining (D) and Western blotting (E) detected the expression of the recombinant vector. Lane 1, whole lysate of untransfected E. coli BL21, as a negative control; Lane 2, whole lysate of pETDuet-hSiglec-9 Fab03-transfected E. coli; Lane 3, supernatant of sonicated lysate of pETDuet-hSiglec-9 Fab03-transfected E. coli; Lane 4, sediment of sonicated lysate of pETDuet-hSiglec-9 Fab03-transfected E. coli; Lane M, protein marker. All strains were induced by IPTG overnight. Coomassie blue staining (F) and Western blotting (G) detected the purified Fab fragments. Lane 1, supernatant of sonicated lysate of pETDuet-hSiglec-9 Fab03-transfected E. coli induced by IPTG overnight; lane M, protein marker. (H) Coomassie blue staining showed that the hS9-Fab03 was expressed, and the heavy chain Fd and light chain L were linked together. (I) horseradish-peroxidase-conjugated goat anti-human antibody (Fab specific) was used to detect the heavy chain Fd, and light chain L of the hS9-Fab03 was expressed and separated in Western blotting. Coomassie blue staining (J) and Western blotting (K) detected the efficiency of hS9-Fab03. Lane 1, supernatant of sonicated lysate of the pETDuet-hSiglec-9 Fab03-transfected E. coli was purified by the His-trap Lambda Fab Select column; lane 2, whole lysate of pETDuet-hSiglec-9 Fab03-transfected E. coli induced by IPTG overnight; lane M, protein marker.