Abstract
Cardiovascular disease (CVD) is the leading cause of death among women in the United States. Endothelial dysfunction and arterial stiffness increase with advancing age and are early predictors of future CVD outcomes. We designed the Modulating Oxidative Stress and Inflammation in Elders (MOXIE) study to examine the effects of 100% watermelon juice as a “food-first” intervention to reduce CVD risk among African American (AA) and European American (EA) women aged 55–69 years. Vascular dysfunction is more pronounced in AA compared to EA women due in part to lower nitric oxide bioavailability caused by higher oxidative stress. However, bioactive compounds in watermelon may improve vascular function by increasing nitric oxide bioavailability and antioxidant capacity. This trial will use a randomized, placebo-controlled, crossover design to investigate the potential of 100% watermelon juice to positively impact various robust measures of vascular function as well as serum biomarkers of oxidative stress and antioxidant capacity. This nutrition intervention and its unique methodology to examine both clinical and mechanistic outcomes are described in this article.
Keywords: Watermelon, lycopene, cardiovascular disease, oxidative stress
Introduction
The Modulating Oxidative Stress and Inflammation in Elders (MOXIE) study is a randomized, double-blind, placebo-controlled crossover trial designed to evaluate the influence of bioactive compounds in 100% watermelon juice on biomarkers of vascular dysfunction and oxidative stress in postmenopausal women. Several natural ingredients in watermelon may act in synergy to improve vascular endothelial function and ameliorate arterial stiffness, two early independent predictors of cardiovascular disease (CVD) (1, 2). Traditionally, primary prevention strategies have targeted men (6). However, CVD accounts for the deaths of one in four women, and it is the leading cause of death for both African American (AA) and European American (EA) women (3). Disparities also exist such that rates of CVD are higher among AA compared to EA women (4). The U.S. Census Bureau predicts that the older adult population will continue to increase and become more racially/ ethnically diverse (7), so interventions to alleviate these gender/ethnic health disparities are a public health priority.
Decline in vascular function with advancing age is a key contributor to development of atherosclerosis, arteriosclerosis, and future cardiovascular events (2, 8, 9). In particular, multiple studies have demonstrated decreased endothelium-dependent vasodilation (9–11) and increased arterial stiffness (12–14) with aging. These age-related changes are due largely to oxidative stress levels (15–17) and reduced bioavailable nitric oxide (2, 9, 11). Research has shown that impaired vascular function is more pronounced in AA compared to EA (18, 19), and clinical studies using nanosensor techniques have revealed that AA women have lower nitric oxide bioavailability, attributable to higher oxidative stress (20). Additional studies examining biomarkers of oxidative stress have also confirmed lower antioxidant capacity in AA compared to EA women (21, 22).
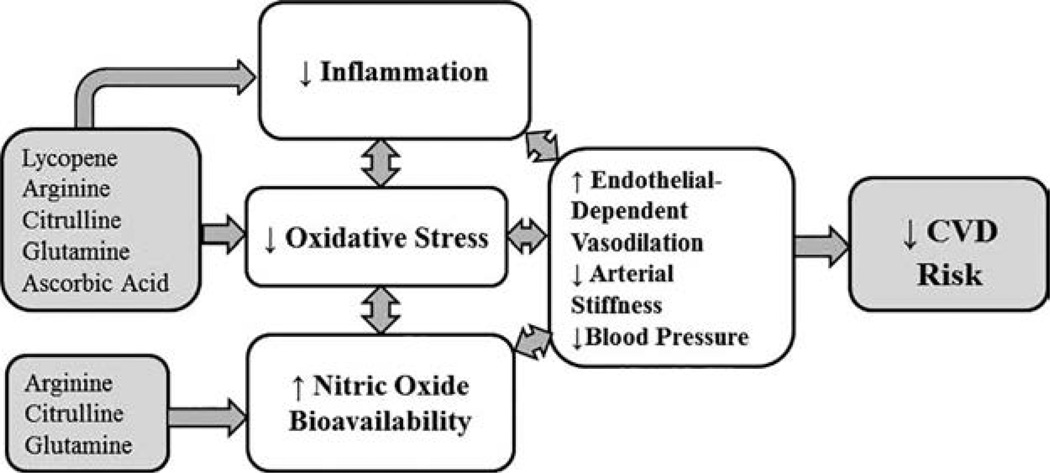
Several bioactive food compounds that are naturally found in watermelon possess properties that may reduce oxidative stress while simultaneously increasing bioavailable nitric oxide. Lycopene is one such lipophilic carotenoid with potent antioxidant properties (23, 24). No Recommended Dietary Allowance (RDA) has been established for this carotenoid even though lycopene cannot be synthesized endogenously and must be consumed in the diet (25). Previous studies indicate that lycopene may beneficially influence multiple underlying mechanisms and clinical outcomes related to CVD. For example, an independent inverse association between circulating lycopene and arterial stiffness has been described (26). Lycopene also appears to inhibit the rate-limiting enzyme in cholesterol synthesis (27) and inhibit the oxidation of low density lipoprotein (LDL) -cholesterol (26). In addition to its own primary antioxidant capacity, lycopene has also been shown to increase the activity of other endogenous antioxidant enzymes in the body (24).
Arginine is another key bioactive food compound found in watermelon that may improve vascular function (28–30). Arginine is a precursor for nitric oxide, the principal vasodilatory molecule produced by endothelial cells (31). Nitric oxide is produced from arginine in a reaction catalyzed by the enzyme endothelial nitric oxide synthase (eNOS) (32). Studies have shown that eNOS is upregulated in endothelial cells of older adults (11, 33) and AA women (20, 34) as an apparent compensatory attempt to generate more nitric oxide. Thus, increasing dietary intake of arginine may improve nitric oxide bioavailability by providing additional substrate for nitric oxide synthesis (35, 36). Independent of nitric oxide pathways, arginine also suppresses generation of reactive oxygen species and attenuates pro-inflammatory cytokines (35–37). Additionally, limited evidence suggests that arginine may decrease total serum cholesterol and LDL cholesterol without impacting HDL cholesterol (38).
The amino acid citrulline was first discovered in watermelon (Citrullus vulgaris) (39), and watermelon is the richest food source of citrulline. Citrulline is converted to arginine in the kidneys (40). It is well-absorbed from the gastrointestinal tract and reaches systemic circulation with little metabolism by the gut or liver (40, 41). Consequently, studies have shown that supplemental citrulline can increase circulating levels of arginine even more than supplemental arginine (41, 42).
In addition to lycopene, arginine, and citrulline, watermelon is also a good food source of glutamine and ascorbic acid, both of which display antioxidant properties (43, 44). Further, ascorbic acid has been shown to enhance arterial dilation through its effect on nitric oxide release (45). Thus, it is plausible that these bioactive food compounds may act in synergy to reduce endothelial dysfunction and arterial stiffness; however, most studies to date have only examined these compounds individually as dietary supplements rather than together in the food matrix. The only studies that have examined the effects of watermelon on vascular function support the notion that this functional food may improve blood pressure and arterial stiffness (46–51), but these studies have been limited to powdered watermelon extracts rather than the whole food.
According to the Healthy Eating Index, adults aged 60 years and older, particularly AA, fall far short of the recommended intake of 2.5 servings of fruit per day (52). Barriers to consumption include edentulism and the hassle of preparing fresh fruits in the home environment (53, 54). Because, one cup of 100% fruit juice counts as one serving of whole fruit according to the 2015 Dietary Guidelines for Americans (55), 100% watermelon juice may provide a way for elders to easily meet dietary recommendations for fruit servings while ingesting beneficial bioactive food compounds capable of improving vascular function. The MOXIE study will be the first to examine the impact of regular intake of 100% watermelon juice on vascular function using a randomized, double-blind, placebo-controlled crossover design. Mechanistic outcomes related to oxidative stress and antioxidant capacity will be assessed alongside clinical measures of vascular health. Examining differences in these outcomes between AA and EA women will shed light on the underpinnings of ethnic disparities related to CVD, and results of this study will help determine whether incorporating this functional food into one’s usual diet may be a practical efficacious way to reduce CVD risk. The protocol along with detailed descriptions of the methodology are presented in this manuscript.
Methodology
Eligibility screening
A total of 17 AA and 17 EA community-dwelling, postmenopausal women aged 55–69 years will be recruited by a variety of advertisement and word-of-mouth methods. Flyers will be placed in various places around the community where they may be seen by members of the target population. These include local YMCAs and other health clubs, libraries, senior centers, churches, shopping centers, and coffee shops. The principal investigators will also work with university-approved media outlets such as local newspapers and evening news programs to advertise the study, and study information will be distributed to community liaisons through the University of Alabama’s Division of Community Affairs.
Inclusion and exclusion criteria are described in Table 1. Study staff will conduct an initial telephone screening with each potential participant. Age and race will be ascertained by self-report with assertion that both parents share the same race as the participants. Postmenopausal status (natural or surgical) will also be affirmed by self-report of no menses for the previous 12 months. Exclusion criteria include obesity (defined by body mass index of 30 kg/m2 or above), weight change greater than 10% over the past year, food allergies, cognitive impairment, and diagnosis of any terminal disease. Because of hepatic and renal metabolism of the amino acids in watermelon (56), women with any history of liver disease or chronic kidney disease will also be excluded. Smoking or other tobacco use, cardiac arrhythmias, artificial pacemaker or defibrillator devices, diabetes, history of hypertension, or any previous cardiac events or procedures will preclude enrollment as these may affect the outcomes to be measured. Likewise, use of medications known to impact endothelial function and arterial stiffness (anticoagulants, cholesterol-lowering medications, and antihypertensive agents) will also be considered among the exclusion criteria.
Table 1.
Inclusion/exclusion criteria.
Inclusion Criteria
|
Exclusion Criteria
|
Respondents deemed eligible and interested from the pre-screening will be provided with a copy of the informed consent to review before an initial screening visit where the study coordinator will explain the protocol in detail, show participants the testing equipment that will be used to assess clinical outcomes, and obtain written consent. Height, weight, and blood pressure will be recorded at the screening visit. Study staff will also administer the Folstein’s Mini-Mental State Exam (57) and verify information collected during the pre-screening interview. Verification of medications and dosages will be assessed by a “brown bag review” of all medications and dietary supplements the participant brings to the screening visit (58). This study has been approved by Institutional Review Boards of the University of Alabama and the DCH Health System.
Components of the intervention
A detailed timeline of the intervention is presented in Table 2. Following a one-week run-in of a controlled-lycopene diet, participants will be randomized to two 12-ounce servings of 100% watermelon juice per day or a calorically similar placebo beverage for four weeks each with a two-week washout period in between.
Table 2.
All studv Drocedures.
| Run-in |
4-Week Regimen of Juice or Placebo |
2-Week Washout |
4-Week Regimen of Juice or Placebo |
||
|---|---|---|---|---|---|
| Days 0–7 | Beginning of Week 1 | End of Week 4 | Weeks 5 & 6 | Beginning of Week 7 | End of Week 10 |
| 3-day food diary | Fasting blood sample | Fasting blood sample | 3-day food diary | Fasting blood sample | Fasting blood sample |
| Height and Weight | Fasting urine sample | Fasting urine sample | Fasting urine sample | Fasting urine sample | |
| FFQ | BIA | BIA | BIA | BIA | |
| FMD | FMD | FMD | FMD | ||
| PWA/PWV | PWA/PWV | PWA/PWV | PWA/PWV | ||
| 24-hr ABPM/HRV | 24-hr ABPM/HRV | 24-hr ABPM/HRV | 24-hr ABPM/HRV | ||
| Physical activity questionnaire | Physical activity questionnaire | Physical activity questionnaire | Physical activity questionnaire | ||
Weekly: Weight, Blood Pressure, Adherence.
FFQ = food frequency questionnaire; BIA = bioelectrical impedance analysis; FMD = flow-mediated dilation; PWA= pulse wave analysis; PWV = pulse wave velocity; ABPM = 24-hour ambulatory blood pressure monitoring; HRV = heart rate variability.
Randomization
Participants will be randomized to the order in which they receive the juice versus the placebo, with randomization stratified by race. A blocked randomization has been generated using PROC PLAN in SAS (version 9.4, Cary, NC). The closed envelope method will be used to assign a randomization code (101–134) to each sequential subject. Bottles of juice and placebo will be labeled with an appropriate code, and only research staff not involved in data collection or analysis will have access to the randomization scheme. The double-blind design will ensure that neither the participant nor the investigators know which beverage they have received in each arm.
Juice and placebo beverages
During each four-week treatment arm, juice or placebo will be provided in 12-ounce opaque bottles, and participants will be asked to consume one bottle in the morning and another in the evening with a meal containing dietary lipid. Based on previous studies (39, 59) two 12-ounce servings of 100% watermelon juice will provide approximately 1 gram of L-citrulline and 32 mg of lycopene. Pasteurized watermelon juice for this study will be supplied by Frey Farms (Keenes, IL) from a single harvest of Estrella variety melons. Samples from each batch of watermelon juice supplied by Frey Farms will be tested for quality control purposes allowing for the study to determine deviations from the mean content of each bioactive compound in watermelon juice.
The placebo beverage will be prepared and bottled in the University of Alabama Food Science Laboratory and will contain water, sucrose, non-nutritive watermelon flavoring, plant-based fibers (pectin and sheared cellulose), malic acid, and Food and Drug Administration–approved food coloring. Taste and acidity of the placebo beverage will be matched to the 100% juice. Pilot testing using SIMS Sensory Quality Panel Software Systems (Berkeley Heights, New Jersey) has confirmed that the 100% watermelon juice and the placebo beverage are comparable in hedonic characteristics of aroma, texture, and taste (data not shown).
One week prior to the intervention, during both treatment periods, and during the washout period, participants will be asked to consume their typical diet of choice with the exception of foods high in lycopene. A list of lycopene-rich foods such as watermelon and tomato products will be provided, and participants will be instructed to limit these foods to two servings per day for the duration of the study. Participants will also be asked to hold all vitamins, minerals, and other dietary supplements during this time. Dietary intake will be closely monitored by three-day food diaries submitted during the one-week run-in period and the two-week washout period as well as by three unannounced 24-hour diet recalls by phone during each treatment arm.
Monitoring of adherence
Participants will report to campus each week to obtain a week’s supply of beverage. During these visits and phone calls with study personnel, participants will be queried about adherence to the protocol and acceptability of the beverages. Adherence will be assessed by log forms with check-off boxes for each dose and random 24-hour diet recalls. Minimal adherence will be defined as 70% of recommended supplementation of two doses per day for four weeks.
Outcome measures
The MOXIE study will evaluate the influence of bioactive compounds in 100% watermelon juice on a variety of outcome measures pertaining to vascular health. The specific aims of this study examine both mechanistic and clinical outcomes, but the aims are not contingent on each other.
Specific aim #1—mechanistic
To determine whether four-week consumption of two 12-ounce servings of 100% watermelon juice per day versus placebo will result in:
increased circulating levels of serum lycopene, citrulline, and arginine
improved serum biomarkers of antioxidant status
decreased urinary biomarkers of oxidative stress
decreased circulating biomarkers of inflammation
Hypotheses: Four-week dietary supplementation with 100% watermelon juice will result in increased antioxidant capacity and decreased inflammation, related to increased serum lycopene, citrulline, and arginine.
To our knowledge, no previous studies have examined the effects of watermelon or watermelon extracts on circulating lycopene. Circulating levels of lycopene, arginine, and citrulline in serum will be assessed using an ultra-high-performance liquid chromatography system (UPLC) (Waters, Milford, MA) equipped with a photo-diode array detector (PDA). UPLC-PDA method conditions will be adapted from previously validated methods (60). Data will be collected and processed using Empower chromatographic software (Waters, Milford, MA). Previous studies have shown that another carotenoid, beta-carotene, can be adversely affected by exposure to light (61). Because it is not known whether the carotenoid lycopene may be similarly affected, care will be taken during serum preparation to wrap vacutainer tubes in aluminum foil, and serum aliquots will be stored in opaque boxes.
In addition to circulating arginine, serum samples will also be analyzed to quantify asymmetrical dimethylarginine (ADMA). ADMA is an endogenous inhibitor of nitric oxide synthase that could potentially decrease nitric oxide synthesis by displacing arginine (62). Studies have shown that ADMA levels increase with aging (32). ADMA in serum samples will be quantified by enzyme-linked immunosorbent assay (Enzo Life Sciences, Farmingdale, NY).
The antioxidant capacity of whole and deproteinated serum will be measured using the oxygen radical absorbance (ORAC) assay on a FLUOstar Optima plate reader (BMG Labtech, Offenburg, Germany) in accordance with the method by Prior and colleagues (63). Serum will be deproteinated according to a published method by the principal investigator using methanol/acetonitrile/acetone (1:1:1, v/v/v) added to serum in a ratio of 1:4 (v/v) (64).
Malondialdehyde (MDA), a product of lipid peroxidation, is a biomarker of oxidative stress. An index of MDA can be measured in urine samples using a thiobarbituric acid-reacting substances (TBARS) assay (65). The TBARS assay will be applied to urine samples to determine whether the intervention ameliorates this biomarker of oxidative stress.
Oxidative stress and chronic low-grade inflammation have been shown to be interrelated in several chronic diseases (66). Tumor necrosis factor-α, interleukin-6, interleukin-8, interleukin-10, and C-reactive protein are serum cytokines commonly measured as biomarkers of inflammation. Thus, tumor necrosis factor-α, interleukin-6, interleukin-8, and interleukin-10 will be assessed by high-sensitivity enzyme immunoassay (EIA; R&D Systems, Minneapolis, MN and ALPCO Diagnostics, Salem, NH).
Specific aim #2—clinical
To determine whether four-week consumption of two 12-ounce servings of 100% watermelon juice per day versus placebo will result in:
improved vascular function as assessed by flow-mediated dilation (FMD), pulse wave analysis (PWA), pulse wave velocity, and 24-hour ambulatory blood pressure monitoring (ABPM).
decreased LDL oxidation
Hypotheses: Four-week dietary supplementation with 100% watermelon juice will result in improved vascular endothelial function and decreases in arterial stiffness, blood pressure, and LDL oxidation.
Blood pressure will be measured in the nondominant arm after five minutes of rest in the supine position using an automated monitor (Mobil-O-Graph, IEM, Stolberg, Germany) with the arm supported at heart level. An appropriate sized cuff is used with a cuff bladder encircling at least 80% of the arm. Three readings are taken at intervals of two minutes, and the second and third readings are averaged. Blood pressure measurements are performed according to published guidelines (67).
FMD is a well-validated method of measuring nitric oxide-mediated vasodilation of the brachial artery. With the participant in a rested, supine position, the technician uses high-resolution Doppler ultrasound to analyze a cross-section of the brachial artery. Baseline diameter measurements are taken for one minute, and then a blood pressure cuff is placed around the forearm and inflated to 50 mm Hg above resting systolic blood pressure. The cuff remains inflated for five minutes, and then it is rapidly deflated. When the cuff is deflated, the resulting hyperemia causes nitric oxide to be released from the vascular endothelium, which in turn, induces vasodilation of the artery. A second ultrasound recording of the brachial artery is acquired 30 seconds before cuff deflation to two minutes afterward. The five largest diameters after deflation are averaged, and FMD represents the percent change in vessel diameter from baseline average to the peak average dilation, and is calculated utilizing the following equation:
Additionally, 10 mid-artery pulsed Doppler signals are obtained at an < 60 degree angle at baseline and upon immediate cuff release to assess baseline and hyperemic velocity parameters. Parameters are analyzed with edge detection software and Doppler flow analyzer (Vascular Research Tools 6 Software, Medical Imaging Applications, LLC, Coralville, IA).
Pulse wave velocity (PWV) is considered the gold standard for assessment of arterial stiffness (13), representing the time necessary for a pulse wave to travel between two different sites. If arteries are stiff and rigid, the speed at which a pressure wave moves through the vessel is increased (68). PWV can be measured by different methods including use of probes, cuffs, ultrasonography, and magnetic resonance imaging–based approaches (13). A fairly new method uses a cuff-based device to estimate PWV by brachial oscillometry (Mobil-O-Graph, IEM, Stolberg, Germany). The device calculates aortic PWV by obtaining traditional blood pressure readings combined with another pressure waveform recording made while the cuff remains inflated to the participant’s diastolic blood pressure for approximately 10 seconds. Although the device obtains a peripheral recording of pulse waves, it uses an ARCSolver algorithm (Austrian Institute of Technology, Vienna, Austria) to estimate central pressure curves by a transfer function. Static estimation of aortic PWV by the Mobil-O-Graph system has demonstrated good reproducibility, and it has been validated against invasive (intra-aortic catheter) and non-invasive (carotid-femoral applanation tonometry) methods (13, 69, 70).
The Mobil-O-Graph device also provides a measurement of PWA. Similar to PWV, the system applies a mathematical model to oscillometric blood pressure measurements to derive an aortic pressure waveform. Comparison of the first and second systolic peaks of the waveform is used to derive and “augmentation index (AIx)” (14). Although arterial stiffness is only one of several factors that affect AIx (13, 68), a higher AIx is highly correlated with arterial stiffness and is considered a surrogate marker (14, 71). Static measurements of PWV and PWA will be obtained in the clinic under standardized conditions before and after each treatment arm.
The Mobil-O-Graph device will also be used for 24-hourABPM before and after each treatment arm. For a 24-hour period at each of these time points, participants will wear an appropriately sized cuff around the nondominant upper arm. An ABPM monitor connected to the cuff will be worn around the neck in a small pouch. The cuff will inflate every 20 minutes during waking hours and every half hour during sleeping hours. Waking and sleeping hours will be defined for each participant based on self-report (72). Participants will also be advised to refrain from excessive movement while the device is recording a measure. Criteria for successful measurement will be specified as higher than 70% of expected measurements during the 24 hours or at least 20 valid waking measurements and seven valid sleep measurements in accordance with published guidelines (73). In tandem with the ABPM recordings, heart rate variability will also be calculated from the standard deviation of waking and sleeping heart rate values obtained during the 24-hour period (74).
Serum oxidized LDL is a sensitive biomarker of CVD risk (75). Measurement of circulating oxidized LDL will be conducted using an enzyme immunoassay (Cell BioLabs, Inc., San Diego, CA) with the resultant chromophore read at 450 nm with a spectrophotometer (PerkinElmer, Akron, OH).
Diet and physical activity covariates
Dietary intake data will be collected to monitor adherence with the controlled-lycopene diet throughout the study. Participants will record three-day food records during the one-week run-in period and during the two-week washout period. Unannounced 24-hr recalls will also be conducted at three time points during each treatment arm. A single trained staff member will administer each 24-hour recall using the multi-pass method that has been validated for use in older adults (76). Data from 24-hour recalls and three-day food records will be coded and entered into the computerized Nutrition Data System for Research (Nutrition Coordinating Center, Minneapolis, MN, 2015), a software program designed for analysis of food diaries and 24-hour dietary recalls. In addition, participants will complete a validated, semi-quantitative Block Food Frequency Questionnaire (77) at the beginning of the study that will estimate customary intake of the bioactive food compounds of interest over the past year. Before and after each treatment arm, participants will also complete a physical activity questionnaire. The Physical Activity Scale for the Elderly is a validated 10-item questionnaire designed to assess habitual levels of physical activity among adults in this age group (78).
Body composition covariates
At screening, weight and height will be measured with a calibrated scale and a standardized stadiometer. Before and after each treatment arm, percent body fat will be estimated by bioelectrical impedance analysis (BIA) (RJL Systems Inc., Clinton Township, MI). This test uses a mild electric current (50 kHz, 800 µA) that is passed between electrodes positioned on the participant’s upper and lower extremities. Total body water is estimated based on different impedance of the current by different tissues, and total body water, in turn, can be used to estimate fat mass and fat-free mass (79).
Fidelity in design
Given the complexity of outcome measures to be assessed, safeguards to maintain fidelity have been incorporated into the study design (80). Study personnel who will be collecting or analyzing outcome data will remain blinded for the duration of the study. Because the FMD measurement can be operator-dependent (81), one dedicated technician with expertise in vascular ultrasounds will perform all tests. In addition to weekly meetings of the two principal investigators, at least one principal investigator will be present for weekly face-to-face team meetings with the research staff. Role-playing, vignettes, and mock study visits will be used to ensure competency; further, checklists and source documents will be used at all data collection to ensure all appropriate study information is collected. Standardized training materials will be collated into a Manual of Procedures that will be given to all team members. A book of standard operating procedures has been developed specifically for individuals involved with bench laboratory analyses. Laboratory personnel will also keep detailed laboratory notebooks, and they will demonstrate proficiency with each method by one-on-one training and direct observation by the principal investigators.
Statistical analysis and sample size considerations
Baseline differences between AA and EA will be evaluated by independent t-tests to ensure that any differences prior to the intervention do not have an effect on outcomes. Effects of 100% watermelon juice versus placebo on outcomes of interest will be determined by two-way ANOVA with repeated measures (juice vs. placebo) x (baseline vs. week four). Treatment effects will also be determined by mixed models analysis using the PROC MIXED procedure with Tukey’s test post-hoc analysis. All analyses will be performed using SAS, version 9.4, with a two-tailed approach. Given the multiple measures and analysis plan of this study design, the significance level of 0.05 will be adjusted using the Bonferroni adjustment for Type 1 error.
Sample size calculations were conducted using published data for both mechanistic and clinical outcomes of interest for the MOXIE Study. Sample size for mechanistic outcomes were based on lycopene as the bioactive food compound in greatest concentration in watermelon (82, 83). Sample size calculations for clinical outcomes were based on official guidelines from the Brachial Artery Reactivity Task Force; a sample size of 20–30 participants is indicated to detect changes in FMD with a crossover design (84). Assuming an average change in serum lycopene of 100 nmol/L with a standard deviation of change of 25 nmol/L, and a significance level of 5%, we would have 80% power to detect a significant change in lycopene with 15 EA and 15 AA participants. However, 17 participants will be enrolled per group to account for unexpected variance in our study sample, the possibility of a lower magnitude of response due to study design differences, and potential drop-outs.
Justification of the dosage and intervention period
Previous studies investigating compounds of interest on noncardiovascular disease outcomes have shown that two weeks is an optimal washout period (51, 59, 85). An intervention period of four weeks for each arm and a dosage of 720 mL/d were chosen based on a previous crossover study with healthy adults that examined the effects of 780 mL/day of unpasteurized watermelon juice on circulating concentrations of arginine. They reported a 9% increase in fasting plasma arginine after one week and an 11% increase after three weeks (p < 0.01) (59). Additionally, the absorption kinetics of lycopene have been studied in a crossover design in which tomato formulations containing 15 mg lycopene increased plasma lycopene by 40 nmol/L with maximum increase observed in 10–12 hours upon consumption (85). Providing 100% watermelon juice twice daily ensures sustained increases in plasma lycopene throughout the intervention period.
Discussion
Although often under-recognized in women, heart disease is the leading cause of death for both AA and EA women as well as for all adults aged 65 years and older in the United States (27.7%) (3). As the population ages and becomes more ethnically diverse (5), practical lifestyle interventions to reduce CVD risk will become increasingly important. The MOXIE study is a prospective randomized, double-blind, placebo-controlled trial that aims to investigate whether regular consumption of 100% watermelon juice may ameliorate vascular endothelial dysfunction and arterial stiffness in AA and EA women aged 55–69 years.
Endothelial dysfunction increases with advancing age and is considered a major risk factor for CVD and future cardiovascular events (2). However, endothelial dysfunction occurs very early in the process of atherosclerosis, and this early stage is potentially reversible (1, 81). Similarly, arteries become stiffer and less elastic with age (14), and arterial stiffness is an early predictor of future CVD risk (13, 86). By targeting women aged 55–69 years not yet diagnosed with CVD, this intervention aims to address underpinnings of vascular dysfunction at a stage when it may be possible to prevent future cardiac events.
Worsening vascular function with age is due largely to reduced bioavailability of nitric oxide (2, 9, 11) at the arterial endothelium along with reduced capacity to combat oxidative stress (15–17). Several bioactive food compounds naturally found in watermelon may work together to increase bioavailable nitric oxide while reducing oxidative stress and improving antioxidant capacity. AA women have been shown to have even more marked reductions in bioavailable nitric oxide and higher levels of oxidative stress than EA women of the same age (20–22). Thus, although both AA and EA women stand to benefit, this intervention directly addresses mechanisms that underpin the higher prevalence of vascular dysfunction among AA women (18, 19).
Previous studies have examined effects of powdered watermelon extract on CVD biomarkers. Chronic ingestion of watermelon extract has shown promise for reducing total and LDL-cholesterol concentrations in both mice (87) and younger adult subjects (88). Two research groups have reported beneficial effects of powdered watermelon extract on blood pressure and other measures of vascular function among adults with pre-existing obesity, prehypertension or stage 1 hypertension (Table 3) (46, 47, 49–51). Although beneficial effects in these studies were thought to be due to higher circulating levels of arginine, the present study will be the first to elucidate mechanisms of action by a robust battery of laboratory analyses to quantify circulating levels of serum arginine, citrulline, and lycopene along with biomarkers of oxidative stress, antioxidant capacity, and inflammatory status. In short, this is the first study of its kind to evaluate bioactive compounds in 100% watermelon juice supplied together in the food matrix on an array of well-validated clinical outcomes to assess vascular endothelial function, arterial stiffness, and 24-hour ambulatory blood pressure parameters.
Table 3.
Studies investigating the impact of watermelon extract on vascular outcomes.
| Author | Sample Size (N) | Intervention | Vascular Outcome Measures |
|---|---|---|---|
| Sex (% Female) | Dose | ||
| Age |
Duration |
||
| Study Design | Health Status | ||
| Massa et al., 2016 (46) Randomized, double-blind, placebo-controlled, parallel study |
Intervention N: 20 % Female: 50 Age: 48.7 ± 1.9 y Health Status: prehypertensive and hypertensive Placebo N: 20 % Female: 45 Age: 47.4 ± 1.2 y Health Status: prehypertensive and hypertensive |
Intervention: watermelon extract Dose: 4g L-citrulline +2 g L-arginine/d Duration: six weeks |
|
| Figueroa et al., 2011 (47) Randomized, double-blind, placebo-controlled, crossover study |
N:9 % Female: 55 Age: 54 ± 3 y Health Status: prehypertensive |
Intervention: watermelon extract Dose: 2.7 g L-citrulline +1.3 g L-arginine/d Duration: six weeks with four weeks washout between intervention arms |
|
| Figueroa et al., 2012 (49) Randomized, placebo-controlled, crossover study |
N: 14 % Female: 78 Age: 58 ± 1 y Health Status: prehypertensive as well as stage 1 HTN. Women were post-menopausal. |
Intervention: watermelon extract Dose: 4g L-citrulline +2 g L-arginine/d Duration: six weeks with two weeks washout between intervention arms |
|
| Figueroa et al., 2013 (50) Randomized, placebo-controlled, crossover study |
N: 12 % Female: 100 Duration: six weeks with two weeks washout between intervention arms |
Intervention: watermelon extract Dose: 4 a L-citrulline +2 a L-arainine/d Age: mean 57y standard error 1y Health Status: obese, postmenopausal women with stage 1 HTN |
|
| Figueroa et al., 2014 (51) Randomized, double-blind, placebo-controlled crossover study |
N: 13 % Female: 76 Age: 57.4 ± 1.4 y Health Status: hypertensive, obese, sedentary. |
Intervention: watermelon extract Dose: 4q L-citrulline +2 q L-arqinine/d Duration: six weeks with two weeks washout between intervention arms |
|
BP, Blood pressure; PWV, pulse wave velocity; PWA, pulse wave analysis; HR, heart rate; HTN, hypertension.
In addition to advancing our knowledge about how the bioactive food compounds in watermelon act in the body, this trial will systematically determine whether the delivery vehicle of 100% fruit juice is a feasible and well-accepted means of increasing fruit intake among this population. An indicator of health and well-being in the Healthy People 2020 initiative includes “consumption of 2 or more fruits per day” (89). Although watermelon is a popular and palatable fruit, national surveys report that only 27% of American adults aged 60 years and older ingest the recommended fruit servings per day (52). A barrier that precludes consumption is the perceived difficulty of preparing fresh fruits (54). Particularly for older adults who live alone, it may be cumbersome to cut and store a large melon and to consume it before spoilage. However, a previous systematic review suggests that 100% fruit and vegetable juices may be practical vehicles for improving nutrient intake among older adults (90).
Moreover, the selection of pasteurized 100% watermelon juice versus watermelon fruit is based on the fact that pasteurized watermelon juice contains lycopene in its most bioavailable form—cis-lycopene. In the fresh fruit, lycopene exists predominantly in the trans-lycopene form. Upon processing watermelon fruit to its juice form, lycopene is released from its cellular plant matrix resulting in free trans-lycopene, which will undergo isomerization to cis-lycopene upon exposure to heat during juice pasteurization (91). Numerous clinical studies have demonstrated the greater absorption and bioavail-ability of cis- versus trans-lycopene (92–94). Hence, pasteurized food products containing significant amounts of lycopene are considered superior to their fresh fruit counterparts.
This intervention will make important contributions to the fields of food science and aging. By assessing both mechanistic and clinical outcomes related to chronic ingestion of 100% watermelon juice, we will add to the evidence base about how key bioactive food compounds in watermelon (lycopene, arginine, citrulline, and other antioxidants) affect the underlying causes of vascular dysfunction. Practically, the intervention is not only innovative but also impactful in helping elders easily meet dietary recommendations for fruit servings while improving intake of beneficial bioactive food compounds. By enrolling half AA and half EA, we will begin to tease apart ethnic differences in vascular function, thereby addressing an important health disparity. As the population ages and becomes more ethnically diverse, such holistic food-first interventions are needed to reduce the burden of chronic disease and to address the Healthy People 2020 goal of alleviating health disparities among women and minorities (95).
Figure 1.
Watermelon: The MOXIE study intervention.
Take away points.
CVD is the leading cause of death among both EA and AA women in the United States.
Watermelon in its 100% juice form was chosen as a food-first intervention based on the authors’ previous research with arginine (ACE) and serum antioxidant capacity related to dietary intake (KCW). Watermelon contains several key ingredients (arginine, citrulline, lycopene, glutamine, and ascorbic acid) that may act synergistically to improve vascular endothelial function and reduce oxidative stress.
Results of this study will determine whether 100% watermelon juice is a palatable, feasible delivery vehicle for several bioactive food compounds that act in concert to improve vascular function and combat oxidative stress.
Acknowledgments
Amy Ellis, PhD, RD and Kristi Crowe-White, PhD, RD are Co-Principal Investigators who contributed equally to the design and management of this study. As such, they should be considered co-anchor authors on this article. Dr. Ellis’ background as a clinical dietitian and Dr. Crowe-White’s background as a food chemist and dietitian allow for a unique study that examines both clinical and mechanistic outcomes. Tanja Dudenbostel, MD has more than 17 years of experience in cardiovascular imaging and function studies for clinical and research purposes. Dr. Dudenbostel’s expertise has been invaluable in planning for vascular outcome measures to be assessed in this study. Julie Locher, PhD, MSPH is a medical sociologist whose experience in designing nutrition-related interventions for older adults spans over two decades. Dr. Locher’s expertise guided various aspects of the study design including plans for recruitment, participant retention, and maintenance of fidelity. The authors are grateful to Dwight Lewis Jr., PhD, MBA and Jason Parton, PhD for development of the randomization scheme to be used in this study.
Funding
This work is supported by American Heart Association Grant 16MCPRP27260233. Pilot funding from the Academy of Nutrition and Dietetics Foundation/Healthy Aging Practice Group allowed for refinement of methodology to be used in this study. Dr. Locher’s participation is supported by the National Institute on Aging (K07AG043588).
References
- 1.Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241(2):507–532. doi: 10.1016/j.atherosclerosis.2015.05.007. PubMed PMID: 26117398. [DOI] [PubMed] [Google Scholar]
- 2.Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ. You’re only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 2014;29(4):250–264. doi: 10.1152/physiol.00059.2013. PubMed PMID: 24985329; PubMed Central PMCID: PMCPMC4103060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Women and Heart Disease Fact Sheet [Internet] 2014 Accessed at http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_women_heart.htm.
- 4.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. PubMed PMID: 24352519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110(8):1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. PubMed PMID: 22499900; PubMed Central PMCID: PMCPMC3366686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker RC. Cardiology patient page. Heart attack and stroke prevention in women. Circulation. 2005;112(17):e273–e275. doi: 10.1161/CIRCULATIONAHA.105.551341. PubMed PMID: 16246950. [DOI] [PubMed] [Google Scholar]
- 7.Ortman J. [November 18, 2014];An Aging Nation: The Older Population in the United States: U.S. Census Bureau. 2014 Accessed at http://www.census.gov/prod/2014pubs/p25-1140.pdf.
- 8.Yavuz B, Yavuz B, Sener D, Cankurtaran M, Halil M, Ulger Z, et al. Advanced age is associated with endothelial dysfunction in healthy elderly subjects. Gerontology. 2008;54(3):153–156. doi: 10.1159/000129064. PubMed PMID: 18441522. [DOI] [PubMed] [Google Scholar]
- 9.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120(9):357–375. doi: 10.1042/CS20100476. PubMed PMID: 21244363; PubMed Central PMCID: PMCPMC3482987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toda N. Age-related changes in endothelial function and blood flow regulation. Pharmacol Ther. 2012;133(2):159–176. doi: 10.1016/j.pharmthera.2011.10.004. PubMed PMID: 22079549. [DOI] [PubMed] [Google Scholar]
- 11.Brandes R, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66(2):286–294. doi: 10.1016/j.cardiores.2004.12.027. PubMed PMID: 15820197. [DOI] [PubMed] [Google Scholar]
- 12.Steppan J, Barodka V, Berkowitz DE, Nyhan D. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol Res Pract. 2011;2011:263585. doi: 10.4061/2011/263585. PubMed PMID: 21845218; PubMed Central PMCID: PMCPMC3154449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66(3):698–722. doi: 10.1161/HYP.0000000000000033. PubMed PMID: 26160955; PubMed Central PMCID: PMCPMC4587661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. PubMed PMID: 17000623. [DOI] [PubMed] [Google Scholar]
- 15.Donato A, Eskurza I, Silver A, Levy A, Pierce G, Gates P, et al. Direct evidence of endo-thelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100(11):1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. PubMed PMID: 17478731. [DOI] [PubMed] [Google Scholar]
- 16.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83(2):456S–460S. doi: 10.1093/ajcn/83.2.456S. PubMed PMID: 16470012. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Mañas L, El-Assar M, Vallejo S, López-Dóriga P, Solís J, Petidier R, et al. Endo-thelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009;8(3):226–238. doi: 10.1111/j.1474-9726.2009.00466.x. PubMed PMID: 19245678. [DOI] [PubMed] [Google Scholar]
- 18.Abbatecola AM, Chiodini P, Gallo C, Lakatta E, Sutton-Tyrrell K, Tylavsky FA, et al. Pulse wave velocity is associated with muscle mass decline: Health ABC study. Age (Dordr) 2011 doi: 10.1007/s11357-011-9238-0. PubMed PMID: 21479573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loehr LR, Espeland MA, Sutton-Tyrrell K, Burke GL, Crouse JR, Herrington DM. Racial differences in endothelial function in postmenopausal women. Am Heart J. 2004;148(4):606–611. doi: 10.1016/j.ahj.2004.04.032. PubMed PMID: 15459590. [DOI] [PubMed] [Google Scholar]
- 20.Malinski T. Understanding nitric oxide physiology in the heart: a nanomedical approach. Am J Cardiol. 2005;96(7B):13i–24i. doi: 10.1016/j.amjcard.2005.07.029. PubMed PMID: 16226932. [DOI] [PubMed] [Google Scholar]
- 21.Morris AA, Zhao L, Patel RS, Jones DP, Ahmed Y, Stoyanova N, et al. Differences in systemic oxidative stress based on race and the metabolic syndrome: the Morehouse and Emory Team up to Eliminate Health Disparities (META-Health) study. Metab Syndr Relat Disord. 2012;10(4):252–259. doi: 10.1089/met.2011.0117. PubMed PMID: 22385338; PubMed Central PMCID: PMCPMC3449394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopes HF, Morrow JD, Stojiljkovic MP, Goodfriend TL, Egan BM, Stoijiljkovic MP. Acute hyperlipidemia increases oxidative stress more in African Americans than in white Americans. Am J Hypertens. 2003;16(5 Pt 1):331–336. doi: 10.1016/s0895-7061(03)00041-4. PubMed PMID: 12745192. [DOI] [PubMed] [Google Scholar]
- 23.Arab L, Steck S. Lycopene and cardiovascular disease. Am J Clin Nutr. 2000;71(6 Suppl):1691S–1695S. doi: 10.1093/ajcn/71.6.1691S. discussion 6S–7S. PubMed PMID: 10837319. [DOI] [PubMed] [Google Scholar]
- 24.LG W. Lycopene modulation of inflammation: role in disease pathology. In: Preedy WA, editor. Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases. San Diego, CA: Academic Press; 2013. pp. 305–317. [Google Scholar]
- 25.Woodside JV, McGrath AJ, Lyner N, McKinley MC. Carotenoids and health in older people. Maturitas. 2015;80(1):63–68. doi: 10.1016/j.maturitas.2014.10.012. PubMed PMID: 25466302. [DOI] [PubMed] [Google Scholar]
- 26.Kim OY, Yoe HY, Kim HJ, Park JY, Kim JY, Lee SH, et al. Independent inverse relationship between serum lycopene concentration and arterial stiffness. Atherosclerosis. 2010;208(2):581–586. doi: 10.1016/j.atherosclerosis.2009.08.009. PubMed PMID: 19767001. [DOI] [PubMed] [Google Scholar]
- 27.Heber D, Lu QY. Overview of mechanisms of action of lycopene. Exp Biol Med (Maywood) 2002;227(10):920–923. doi: 10.1177/153537020222701013. PubMed PMID: 12424335. [DOI] [PubMed] [Google Scholar]
- 28.Preli RB, Klein KP, Herrington DM. Vascular effects of dietary L-arginine supplementation. Atherosclerosis. 2002;162(1):1–15. doi: 10.1016/s0021-9150(01)00717-1. PubMed PMID: 11947892. [DOI] [PubMed] [Google Scholar]
- 29.Bode-Böger S, Muke J, Surdacki A, Brabant G, Böger R, Frölich J. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med. 2003;8(2):77–81. doi: 10.1191/1358863x03vm474oa. PubMed PMID: 14518608. [DOI] [PubMed] [Google Scholar]
- 30.Monti LD, Casiraghi MC, Setola E, Galluccio E, Pagani MA, Quaglia L, et al. L-arginine enriched biscuits improve endothelial function and glucose metabolism: a pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism. 2013;62(2):255–264. doi: 10.1016/j.metabol.2012.08.004. PubMed PMID: 23040413. [DOI] [PubMed] [Google Scholar]
- 31.Chen F, Lucas R, Fulton D. The subcellular compartmentalization of arginine metabolizing enzymes and their role in endothelial dysfunction. Front Immunol. 2013;4:184. doi: 10.3389/fimmu.2013.00184. PubMed PMID: 23847624; PubMed Central PMCID: PMCPMC3705211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Álvares TS, Meirelles CM, Bhambhani YN, Paschoalin VM, Gomes PS. L-Arginine as a potential ergogenic aid in healthy subjects. Sports Med. 2011;41(3):233–248. doi: 10.2165/11538590-000000000-00000. PubMed PMID: 21395365. [DOI] [PubMed] [Google Scholar]
- 33.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, et al. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297(1):H425–H432. doi: 10.1152/ajpheart.00689.2008. PubMed PMID: 19465546; PubMed Central PMCID: PMCPMC2711733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109(21):2511–2517. doi: 10.1161/01.CIR.0000129087.81352.7A. PubMed PMID: 15159296. [DOI] [PubMed] [Google Scholar]
- 35.Appleton J. Arginine: clinical potential of a semi-essential amino. Altern Med Rev. 2002;7(6):512–522. PubMed PMID: 12495375. [PubMed] [Google Scholar]
- 36.Tong B, Barbul A. Cellular and physiological effects of arginine. Mini Rev Med Chem. 2004;4(8):823–832. doi: 10.2174/1389557043403305. PubMed PMID: 15544543. [DOI] [PubMed] [Google Scholar]
- 37.Potenza M, Nacci C, Mitolo-Chieppa D. Immunoregulatory effects of L-arginine and therapeutical implications. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1(1):67–77. doi: 10.2174/1568008013341811. PubMed PMID: 12476782. [DOI] [PubMed] [Google Scholar]
- 38.Hurson M, Regan MC, Kirk SJ, Wasserkrug HL, Barbul A. Metabolic effects of arginine in a healthy elderly population. JPEN J Parenter Enteral Nutr. 1995;19(3):227–230. doi: 10.1177/0148607195019003227. PubMed PMID: 8551652. [DOI] [PubMed] [Google Scholar]
- 39.Jayaprakasha G, KN M, Patil B. Rapid HPLC-UV method for quantification of L-citrulline in watermelon and its potential role on smooth muscle relaxation markers. Food Chemistry. 2011;127:240–248. [Google Scholar]
- 40.Bahri S, Zerrouk N, Aussel C, Moinard C, Crenn P, Curis E, et al. Citrulline: from metabolism to therapeutic use. Nutrition. 2013;29(3):479–484. doi: 10.1016/j.nut.2012.07.002. PubMed PMID: 23022123. [DOI] [PubMed] [Google Scholar]
- 41.Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A, et al. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol. 2008;65(1):51–59. doi: 10.1111/j.1365-2125.2007.02990.x. PubMed PMID: 17662090; PubMed Central PMCID: PMCPMC2291275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moinard C, Maccario J, Walrand S, Lasserre V, Marc J, Boirie Y, et al. Arginine behaviour after arginine or citrulline administration in older subjects. Br J Nutr. 2016;115(3):399–404. doi: 10.1017/S0007114515004638. PubMed PMID: 26619904. [DOI] [PubMed] [Google Scholar]
- 43.Meynial-Denis D. Glutamine metabolism in advanced age. Nutr Rev. 2016;74(4):225–236. doi: 10.1093/nutrit/nuv052. PubMed PMID: 26936258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roth E. Nonnutritive effects of glutamine. J Nutr. 2008;138(10):2025S–2031S. doi: 10.1093/jn/138.10.2025S. PubMed PMID: 18806119. [DOI] [PubMed] [Google Scholar]
- 45.Plantinga Y, Ghiadoni L, Magagna A, Giannarelli C, Franzoni F, Taddei S, et al. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens. 2007;20(4):392–397. doi: 10.1016/j.amjhyper.2006.09.021. PubMed PMID: 17386345. [DOI] [PubMed] [Google Scholar]
- 46.Massa NM, Silva AS, Toscano LT, Silva JD, Persuhn DC, Gonçalves MD. Watermelon extract reduces blood pressure but does not change sympathovagal balance in prehypertensive and hypertensive subjects. Blood Press. 2016;25(4):1–5. doi: 10.3109/08037051.2016.1150561. PubMed PMID: 26947668. [DOI] [PubMed] [Google Scholar]
- 47.Figueroa A, Sanchez-Gonzalez MA, Perkins-Veazie PM, Arjmandi BH. Effects of watermelon supplementation on aortic blood pressure and wave reflection in individuals with prehypertension: a pilot study. Am J Hypertens. 2011;24(1):40–44. doi: 10.1038/ajh.2010.142. PubMed PMID: 20616787. [DOI] [PubMed] [Google Scholar]
- 48.Figueroa A, Sanchez-Gonzalez M, Wong A, Arjmandi B. Arterial stiffness and blood pressure are reduced after watermelon supplementation in obese with prehypertension and hypertension. The FASEB Journal. 2012;26:385. [Google Scholar]
- 49.Figueroa A, Sanchez-Gonzalez MA, Wong A, Arjmandi BH. Watermelon extract supplementation reduces ankle blood pressure and carotid augmentation index in obese adults with prehypertension or hypertension. Am J Hypertens. 2012;25(6):640–643. doi: 10.1038/ajh.2012.20. PubMed PMID: 22402472. [DOI] [PubMed] [Google Scholar]
- 50.Figueroa A, Wong A, Hooshmand S, Sanchez-Gonzalez MA. Effects of watermelon supplementation on arterial stiffness and wave reflection amplitude in postmenopausal women. Menopause. 2013;20(5):573–577. doi: 10.1097/GME.0b013e3182733794. PubMed PMID: 23615650. [DOI] [PubMed] [Google Scholar]
- 51.Figueroa A, Wong A, Kalfon R. Effects of watermelon supplementation on aortic hemo-dynamic responses to the cold pressor test in obese hypertensive adults. Am J Hypertens. 2014;27(7):899–906. doi: 10.1093/ajh/hpt295. PubMed PMID: 24572702. [DOI] [PubMed] [Google Scholar]
- 52.Ervin R. [cited 2014 November 18, 2014];Healthy Eating Index scores among adults, 60 years of age and over: Centers for Disease Control and Prevention (CDC) 2008 Accessed at http://www.cdc.gov/nchs/data/ad/ad395.pdf.
- 53.Emami E, de Souza RF, Kabawat M, Feine JS. The impact of edentulism on oral and general health. Int J Dent. 2013;2013:498305. doi: 10.1155/2013/498305. PubMed PMID: 23737789; PubMed Central PMCID: PMCPMC3664508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Locher JL, Ritchie CS, Roth DL, Sen B, Vickers KS, Vailas LI. Food choice among home-bound older adults: motivations and perceived barriers. J Nutr Health Aging. 2009;13(8):659–664. doi: 10.1007/s12603-009-0194-7. PubMed PMID: 19657547; PubMed Central PMCID: PMCPMC2749957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.United States Department of Agriculture. 2015 Dietary Guidelines for Americans [Internet] 2016 Accessed at health.gov/dietaryguidelines/2015/guidelines.
- 56.Böger RH. The pharmacodynamics of L-arginine. Altern Ther Health Med. 2014;20(3):48–54. PubMed PMID: 24755570. [PubMed] [Google Scholar]
- 57.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. PubMed PMID: 1512391. [DOI] [PubMed] [Google Scholar]
- 58.Weiss BD, Brega AG, LeBlanc WG, Mabachi NM, Barnard J, Albright K, et al. Improving the effectiveness of medication review: guidance from the Health Literacy Universal Precautions Toolkit. J Am Board Fam Med. 2016;29(1):18–23. doi: 10.3122/jabfm.2016.01.150163. PubMed PMID: 26769873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collins JK, Wu G, Perkins-Veazie P, Spears K, Claypool PL, Baker RA, et al. Watermelon consumption increases plasma arginine concentrations in adults. Nutrition. 2007;23(3):261–266. doi: 10.1016/j.nut.2007.01.005. PubMed PMID: 17352962. [DOI] [PubMed] [Google Scholar]
- 60.Mao HM, Wei W, Xiong WJ, Lu Y, Chen BG, Liu Z. Simultaneous determination of l-citrulline and l-arginine in plasma by high performance liquid chromatography. Clin Biochem. 2010;43(13–14):1141–1147. doi: 10.1016/j.clinbiochem.2010.05.017. PubMed PMID: 20540937. [DOI] [PubMed] [Google Scholar]
- 61.Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8(1):113–117. doi: 10.1021/pr800545q. PubMed PMID: 19072545; PubMed Central PMCID: PMCPMC2655764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilcken DE, Sim AS, Wang J, Wang XL. Asymmetric dimethylarginine (ADMA) in vascular, renal and hepatic disease and the regulatory role of L-arginine on its metabolism. Mol Genet Metab. 2007;91(4):309–317. doi: 10.1016/j.ymgme.2007.04.017. discussion 8 PubMed PMID: 17560156. [DOI] [PubMed] [Google Scholar]
- 63.Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, Howard L, et al. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J Agric Food Chem. 2003;51(11):3273–3279. doi: 10.1021/jf0262256. PubMed PMID: 12744654. [DOI] [PubMed] [Google Scholar]
- 64.Crowe KM. Optimizing protein precipitation efficiency for assessing the contribution of low molecular weight compounds to serum antioxidant capacity. Clin Biochem. 2014;47(15):116–118. doi: 10.1016/j.clinbiochem.2014.06.021. PubMed PMID: 24997420. [DOI] [PubMed] [Google Scholar]
- 65.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. PubMed PMID: 36810. [DOI] [PubMed] [Google Scholar]
- 66.Biswas SK. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016;2016:5698931. doi: 10.1155/2016/5698931. PubMed PMID: 26881031; PubMed Central PMCID: PMCPMC4736408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. PubMed PMID: 15699287. [DOI] [PubMed] [Google Scholar]
- 68.Davies JI, Struthers AD. Pulse wave analysis and pulse wave velocity: a critical review of their strengths and weaknesses. J Hypertens. 2003;21(3):463–472. doi: 10.1097/00004872-200303000-00004. PubMed PMID: 12640232. [DOI] [PubMed] [Google Scholar]
- 69.Aissopou EK, Argyris AA, Nasothimiou EG, Konstantonis GD, Tampakis K, Tentolouris N, et al. Ambulatory aortic stiffness is associated with narrow retinal arteriolar caliber in hypertensives: the SAFAR Study. Am J Hypertens. 2016;29(5):626–633. doi: 10.1093/ajh/hpv145. PubMed PMID: 26304958. [DOI] [PubMed] [Google Scholar]
- 70.Baumann M, Wassertheurer S, Suttmann Y, Burkhardt K, Heemann U. Aortic pulse wave velocity predicts mortality in chronic kidney disease stages 2–4. J Hypertens. 2014;32(4):899–903. doi: 10.1097/HJH.0000000000000113. PubMed PMID: 24609217. [DOI] [PubMed] [Google Scholar]
- 71.Stoner L, Young JM, Fryer S. Assessments of arterial stiffness and endothelial function using pulse wave analysis. Int J Vasc Med. 2012;2012:903107. doi: 10.1155/2012/903107. PubMed PMID: 22666595; PubMed Central PMCID: PMCPMC3361177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163(9):691–700. doi: 10.7326/M15-1270. PubMed PMID: 26457954; PubMed Central PMCID: PMCPMC4638406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O’Brien E, Parati G, Stergiou G. Ambulatory blood pressure measurement: what is the international consensus? Hypertension. 2013;62(6):988–994. doi: 10.1161/HYPERTENSIONAHA.113.02148. PubMed PMID: 24060895. [DOI] [PubMed] [Google Scholar]
- 74.Dudenbostel T, Acelajado MC, Pisoni R, Li P, Oparil S, Calhoun DA. Refractory hypertension: evidence of heightened sympathetic activity as a cause of antihypertensive treatment failure. hypertension. 2015;66(1):126–133. doi: 10.1161/HYPERTENSIONAHA.115.05449. PubMed PMID: 25987662; PubMed Central PMCID: PMCPMC4465856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Itabe H, Obama T, Kato R. The dynamics of oxidized LDL during atherogenesis. J Lipids. 2011;2011:418313. doi: 10.1155/2011/418313. PubMed PMID: 21660303; PubMed Central PMCID: PMCPMC3108093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harnack L, Stevens M, Van Heel N, Schakel S, Dwyer JT, Himes J, editors. A computer-based approach for assessing dietary supplement use in conjunction with dietary recalls. J Food Comp Anal. 2008;21(Suppl 1):S78–S82. doi: 10.1016/j.jfca.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–1335. doi: 10.1016/0895-4356(90)90099-b. PubMed PMID: 2254769. [DOI] [PubMed] [Google Scholar]
- 78.Washburn R, McAuley E, Katula J, Mihalko S, Boileau R. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52(7):643–651. doi: 10.1016/s0895-4356(99)00049-9. PubMed PMID:10391658. [DOI] [PubMed] [Google Scholar]
- 79.Kyle U, Bosaeus I, De Lorenzo A, Deurenberg P, Elia M, Manuel Gómez J, et al. Bioelec-trical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23(6):1430–1453. doi: 10.1016/j.clnu.2004.09.012. PubMed PMID: 15556267. [DOI] [PubMed] [Google Scholar]
- 80.Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23(5):443–451. doi: 10.1037/0278-6133.23.5.443. PubMed PMID: 15367063. [DOI] [PubMed] [Google Scholar]
- 81.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55(5):1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. PubMed PMID: 20351340; PubMed Central PMCID: PMCPMC2878744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaneley R, Nieman D, Knab A, Perkins-Veazie P, Henson D, Ciadella-Kam L, et al. Comparison of watermelon and carbohydrate beverage on exercise-induced oxidative stress, inflammation, and immune dysfunction, and augmentation index. The FASEB Journal. 2013;27:1076. [Google Scholar]
- 83.Jacob K, Periago MJ, Böhm V, Berruezo GR. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Br J Nutr. 2008;99(1):137–146. doi: 10.1017/S0007114507791894. PubMed PMID: 17640421. [DOI] [PubMed] [Google Scholar]
- 84.Corretti M, Anderson T, Benjamin E, Celermajer D, Charbonneau F, Creager M, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vaso-dilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. PubMed PMID: 11788217. [DOI] [PubMed] [Google Scholar]
- 85.Riso P, Brusamolino A, Contino D, Martini D, Vendrame S, Del Bo’ C, et al. Lycopene absorption in humans after the intake of two different single-dose lycopene formulations. Pharmacol Res. 2010;62(4):318–321. doi: 10.1016/j.phrs.2010.06.005. PubMed PMID: 20558293. [DOI] [PubMed] [Google Scholar]
- 86.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–448. doi: 10.1097/HJH.0b013e32834fa8b0. PubMed PMID: 22278144. [DOI] [PubMed] [Google Scholar]
- 87.Poduri A, Rateri DL, Saha SK, Saha S, Daugherty A. Citrullus lanatus ‘sentinel’ (watermelon) extract reduces atherosclerosis in LDL receptor-deficient mice. J Nutr Biochem. 2013;24(5):882–886. doi: 10.1016/j.jnutbio.2012.05.011. PubMed PMID: 22902326; PubMed Central PMCID: PMCPMC3504646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Massa NM, Silva AS, de Oliveira CV, Costa MJ, Persuhn DC, Barbosa CV, et al. Supplementation with watermelon extract reduces total cholesterol and LDL cholesterol in adults with dyslipidemia under the influence of the MTHFR C677 T polymorphism. J Am Coll Nutr. 2016;35(6):1–7. doi: 10.1080/07315724.2015.1065522. PubMed PMID: 26934084. [DOI] [PubMed] [Google Scholar]
- 89.Services USDoHaH. Healthy People 2020 Disparities. 2014 Nov 18; Accessed at http://www.healthypeople.gov/2020/about/foundation-health-measures/Disparities.
- 90.Esfahani A, Wong JM, Truan J, Villa CR, Mirrahimi A, Srichaikul K, et al. Health effects of mixed fruit and vegetable concentrates: a systematic review of the clinical interventions. J Am Coll Nutr. 2011;30(5):285–294. doi: 10.1080/07315724.2011.10719971. PubMed PMID: 22081614. [DOI] [PubMed] [Google Scholar]
- 91.Shi J, Le Maguer M. Lycopene in tomatoes: chemical and physical properties affected by food processing. Crit Rev Food Sci Nutr. 2000;40(1):1–42. doi: 10.1080/10408690091189275. PubMed PMID: 10674200. [DOI] [PubMed] [Google Scholar]
- 92.Cohn W, Thürmann P, Tenter U, Aebischer C, Schierle J, Schalch W. Comparative multiple dose plasma kinetics of lycopene administered in tomato juice, tomato soup or lycopene tablets. Eur J Nutr. 2004;43(5):304–312. doi: 10.1007/s00394-004-0476-0. PubMed PMID: 15309451. [DOI] [PubMed] [Google Scholar]
- 93.Re R, Fraser PD, Long M, Bramley PM, Rice-Evans C. Isomerization of lycopene in the gastric milieu. Biochem Biophys Res Commun. 2001;281(2):576–581. doi: 10.1006/bbrc.2001.4366. PubMed PMID: 11181086. [DOI] [PubMed] [Google Scholar]
- 94.Basu A, Imrhan V. Tomatoes versus lycopene in oxidative stress and carcinogenesis: conclusions from clinical trials. Eur J Clin Nutr. 2007;61(3):295–303. doi: 10.1038/sj.ejcn.1602510. PubMed PMID: 16929242. [DOI] [PubMed] [Google Scholar]
- 95.Centers for Disease Control. Healthy People 2020 [Internet] 2016 Accessed at http://www.cdc.gov/aging/agingdata/data-portal/healthy-people.html#HealthyPeopleObjectives.