Abstract
Myeloid-derived suppressor cells (MDSCs) have garnered much attention in recent years as a potential target for altering the immunosuppressive tumor microenvironment in a variety of solid tumor types. The ability to accurately assess the immunosuppressive capacity of MDSCs is fundamental to the development of therapeutic approaches aimed at disabling these immunosuppressive functions. In this article we provide evidence that the use of CD3/28 coated microbeads leads to artefactual T-lymphocyte suppression due to sequestration of beads by MDSCs isolated from the spleens of wild-type mice bearing subcutaneous syngeneic, carcinogen-induced oral cavity carcinomas. Mechanisms of this finding may include early MDSC death and acquisition of phagocytic capacity. These artefactual findings were avoided by eliminating the use of microbeads and instead using plate bound CD3/28 antibody as the T-lymphocyte stimulus. We propose model-specific validation of microbead-based MDSC assays, or use of an alternative stimulation approach such as plate bound CD3/28 antibodies.
Keywords: Myeloid-derived suppressor cells, MDSC, T-cell suppression assay, phagocytosis, artifact, CD3/28 microbeads
1. Introduction
Myeloid-Derived Suppressor Cells (MDSCs) are a heterogeneous population of immunosuppressive myeloid cells that are recruited into solid tumors through chemokine signaling [1]. Elevated numbers of MDSCs in both the tumor and the periphery are correlated with poor outcomes in a variety of cancer types [1–4]. MDSCs mediate immunosuppression through a number of mechanisms, including the release of immunosuppressive cytokines and the depletion of nutrients and metabolites required for T-lymphocyte function [1,5]. Strategies to either block the recruitment or alter the function of MDSCs within the tumor microenvironment (TME) can increase effector T-cell function and improve responses to anti-cancer therapies [6].
To characterize the mechanisms of MDSC-mediated immunosuppression and to evaluate therapies that may reverse this suppression, a reliable assay is required to quantify MDSC function. However, ex vivo evaluation of MDSCs is complicated by their poor survival in culture and tendency to differentiate into mature myeloid cells when cultured in the presence of growth factors such as GM-CSF [7]. A variety of in vitro assays have been used to measure the immunosuppressive capacity of MDSCs. Mixed leukocyte assays evaluating the impact of MDSCs on T-lymphocytes stimulated with anti-CD3/anti-CD28 coated microbeads have become popular due to their relative simplicity and the potency of the CD3/28-mediated T-cell stimulation. In these assays, reduced T-cell proliferation or IFNγ production in the presence of MDSCs has been interpreted as an accurate indication of MDSC suppressive function.
However, concerns in both our lab and others have begun to arise as to the physiologic accuracy and potential for artifact in this polystyrene microbead-based assay [8]. Here, using splenic MDSCs isolated from mice bearing syngeneic, carcinogen-induced oral cavity carcinomas grown subcutaneously in wild-type mice, we demonstrate artefactual suppression of CD3/28 microbead stimulated T-lymphocyte proliferation by MDSCs due to sequestration of beads away from T-lymphocytes in a mixed leukocyte assay. This effect could not be reversed with inhibitors of known MDSC immunosuppressive mechanisms, and was likely due in part to early phagocytic activity and death of sorted peripheral MDSCs. Reversible and dose-dependent inhibition of T-lymphocyte proliferation by MDSCs was achieved with elimination of polystyrene beads from the assay. We propose model-specific validation of microbead-based MDSC assays, or use of an alternative stimulation approach such as plate bound CD3/28 antibodies.
2. Materials and Methods
2.1 Murine tumor model
The murine oral cancer (MOC) model is a carcinogen-induced model of oral cavity cancer that is transplantable into fully immunocompetent C57BL/6 (B6) mice [9]. MOC1 cells were provided by Dr. R. Uppaluri (Washington University School of Medicine). MOC cells were cultured as previously described [10]. All animal experiments were approved by the NIDCD Animal Care and Use Committee (ASP #1364-14). To generate syngeneic tumor-bearing mice, 4×106 MOC1 cells were injected subcutaneously in matrigel into the flank of WT C57BL/6 (B6) mice. Tumors were engrafted and allowed to reach at least 500 mm3 before MDSC isolation.
2.2 Cell sorting
Splenic single cell suspensions were generated from WT B6 or MOC1 tumor-bearing mice through mechanical dissociation and RBC lysis (Biolegend). To isolate responder T-cells, WT B6 splenocytes were stained and sorted on an autoMACS magnetic sorter (Miltenyi Biotec) using the pan T-cell negative selection kit from Miltenyi (#130-095-130) per the manufacturer’s instructions. For MDSC isolation, splenic single cell suspensions were stained with the anti-Ly6G microbead kit from Miltenyi (#130-092-332) per the manufacturer’s instructions and isolated on an autoMACS magnetic sorter.
2.3. Flow cytometry
Cell surface staining was performed using fluorophore conjugated anti-mouse CD4 clone GK1.5, CD8 clone 53-6.7, Gr1 clone RB6-8c5, and CD11b clone M1/70 antibodies from Biolegend. Dead cells were excluded via 7AAD negativity. Data was acquired on a FACSCanto using FACSDiva software (BD Biosciences) and analyzed on FlowJo software vX10.07r2.
2.4 T-Cell proliferation assay
WT B6 T-cells were labeled with 5 μM carboxyfluorescein succinimidyl ester (CFSE, Sigma Aldrich) as previously described [11]. 8×104 CSFE-labelled T-lymphocytes were stimulated with a 1:1 ratio of anti-CD3/anti-CD28 coated dynabeads (ThermoFisher) in round-bottom 96-well plates in the presence of MDSCs as indicated for 3-4 days. For plate-bound CD3/28 stimulation, 5 μg/mL each of anti-CD3 (clone 145-2C11, eBioscience) and anti-CD28 (clone 37.51, eBioscience) was diluted in PBS and coated onto flat-bottom 96-well plates (Corning) overnight at 4°C. CFSE labeled T-cells were co-cultured with the indicated ratios of MDSCs for four hours, then added to the prepared CD3/28 coated plate (wells were washed with PBS × 2 to removed unbound antibody prior to adding cells). Where indicated, MDSCs and T-lymphocytes were exposed to 300 μM of nor-NOHA (arginase inhibitor) or L-NMMA (iNOS inhibitor) for 4 hours before T-lymphocyte stimulation with either CD3/28 microbeads or plate bound antibody. After 3 days in culture, T-cell CFSE peak distribution was quantified by flow cytometry. T-cells and MDSCs were cultured in complete media (RPMI 1640 supplemented with 10% FCS, 1.5% HEPES, 1% glutamine, 1% nonessential amino acids, 1% sodium pyruvate, 1% Pen/Strep, 0.1% gentamycin, 50 μM beta-mercaptoethanol). T-lymphocyte proliferation was quantified as the average number of divisions for all cells in the culture (division index) using FlowJo software. Percept inhibition of proliferation was calculated using the following:
2.5 In vitro cell viability
Viability of MDSCs was measured by staining with acridine orange and propidium iodide (AOPI solution, Nexcelcom) and quantified on a Cellometer Auto 2000 fluorescent imager.
2.6 Phagocytosis assay
Sorted MDSCs were exposed to pHrodo E. coli BioParticles (ThermoFisher P35366) at 100 μg/1×105 cells in live cell imaging solution (ThermoFisher #A14291DJ) for 90 minutes. Conjugated cell surface antibodies were added as indicated for 30 minutes, cells were extensively washed, and cells were analyzed by flow cytometry.
2.7 Cellular photography
Photomicrographs were obtained on an Olympus IX70 inverted light microscope and an Olympus DP72 camera using CellSens Standard 1.5 software. No images were manipulated with editing software.
2.7 Statistical analysis
Tests of significance between pairs of data are reported as p-values, derived using a student’s t-test with a two-tailed distribution with significance set to p<0.05. Comparison of mean values to zero was done using a one-sample t-test. Error bars indicate standard error of the mean (SEM). All analysis was performed using GraphPad Prism v7.
3. Results
3.1 MDSCs appear to be highly immunosuppressive at low dilutions in a CD3/28 microbead-based assay
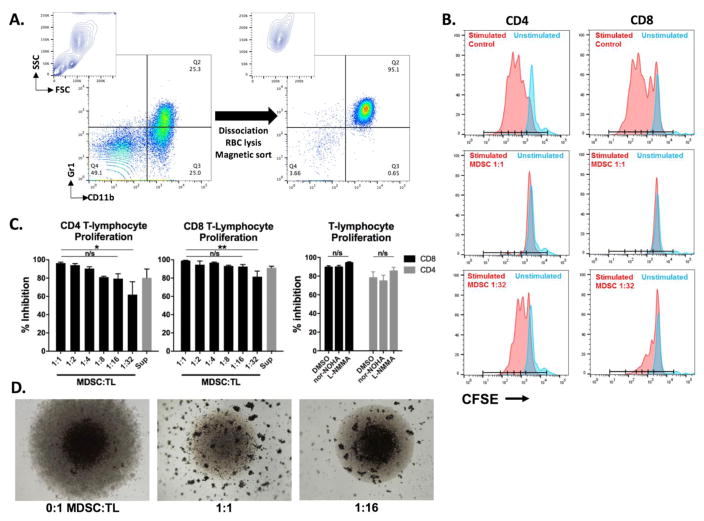
Initial quantification of the immunosuppressive capacity of MDSCs were made using a CD3/28 microbead-based assay. MDSCs were sorted from MOC1 tumor-bearing mice with high purity (Figure 1A) and CFSE distribution, as a measure of T-lymphocyte proliferation, was measured after CD3/28 microbead stimulation for 72 hours (Figure 1B). Interestingly, MDSC:T-cell ratios down to 1:32 demonstrated significant inhibition (p<0.001 for all dilutions tested) of CD4 and CD8 T-cell proliferation in a dilution-dependent fashion (Figure 1C, left panels). This potent suppression of T-lymphocyte proliferation could not be suppressed with nor-NOHA (arginase inhibitor) or L-NMMA (iNOS inhibitor), inhibitors of two well established mechanisms of MDSC immunosuppression (Figure 1C, right panel). However, visual examination of this mixed leukocyte assay demonstrated that while beads pooled in the base of the round bottom well adjacent to and leading to an expansion of T-lymphocytes without MDSCs, the microbeads were spread throughout the culture media in the presence of MDSCs (in apparent aggregates) in a dilution-dependent fashion (Figure 1D).
Figure 1. MDSCs appear highly immunosuppressive at low ratios in bead-based T-lymphocyte proliferation assay.
A, Contour and dot-plots demonstrating high MDSC enrichment from tumor-bearing mouse spleens following mechanical dissociation and magnetic sorting. B, Flow cytometry histograms demonstrating the CFSE distribution of T-cells incubated with MDSCs at the indicated MDSC:T-lymphocyte ratios in the presence of CD3/28 microbeads. Blue peaks are unstimulated CFSE-labelled T-lymphocytes, red peaks are CD3/28 microbead stimulated T-lymphocytes. MDSC supernatant was generated over 24 hours of culture in complete T-cell media where indicated. C left panels, Quantification of CFSE dilution from the experiment shown in B, expressed as percent inhibition of control stimulated T-cells. *, p<0.05; **, p<0.01, ANOVA. Right panel, Sorted MDSCs and CFSE-labelled T-lymphocytes were incubated for 4 hours with 300 μM arginase (nor-NOHA) or iNOS (L-NMMA) inhibitors or an equivalent concentration of DMSO before being combined with CD3/28 microbeads. D, 4x photomicrographs of round-bottom wells containing MDSCs and T-lymphocytes at the indicated ratios after 72 hours of co-incubation. CD3/28 microbeads appear as dense dots, whereas the lighter translucent dots are T-lymphocytes pooled in the base of the round-bottom well. All results shown are representative of a least 5 independent assays. TL, T-lymphocytes. Nor-NOHA, arginase inhibitor; L-NMMA, iNOS inhibitor.
3.2 MDSCs prevent T-lymphocyte stimulation through sequestration of CD3/CD28 microbeads
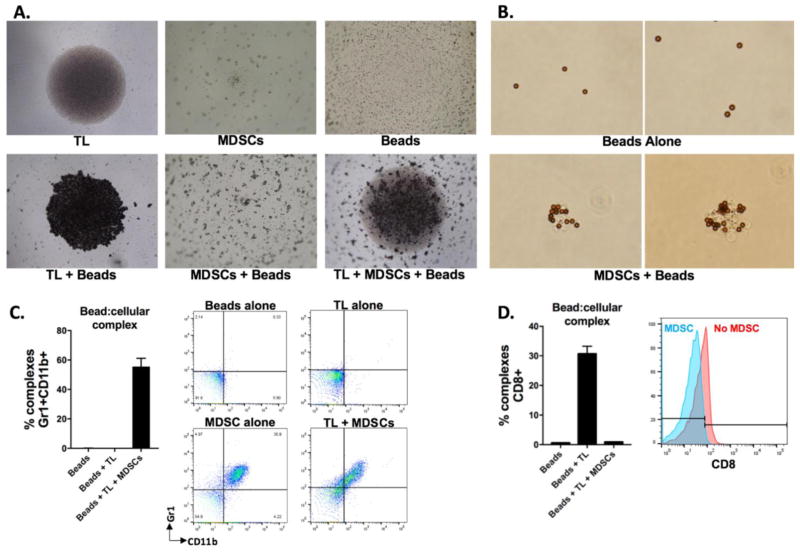
To characterize the behavior of each variable in culture, photomicrographs were taken after 24 hours in culture. T-lymphocytes alone settled to the bottom of the well, whereas clustered MDSCs alone or microbeads alone scattered throughout the well (Figure 2A, top panels). When beads were cultured with T-lymphocytes, they settled to the bottom of the well adjacent to the T-lymphocytes (Figure 2A, bottom panel). Microbeads cultured with MDSCs scattered in clumps throughout the well, mimicking the behavior of MDSCs alone in culture. This scattered microbead/MDSC appearance is maintained in the presence of T-lymphocytes. On closer examination of microbeads magnetically separated from co-culture with MDSCs, microbead:cellular complexes are observed (Figure 2B). To verify this interaction, microbeads were magnetically separated after 3 days in culture with T-lymphocytes alone or in combination with MDSCs, and expression of cellular markers on the microbead:cellular complexes was quantified by flow cytometry. Complexes isolated from culture with T-lymphocytes or beads alone did not express MDSC markers CD11b or Gr1, whereas over half of the microbeads isolated from culture with T-lymphocytes and MDSCs were CD11b+Gr1+, indicating physical contact between microbeads and MDSCs (Figure 2C). Conversely, while microbeads alone bind to CD8 T-lymphocytes, the addition of MDSCs to the culture of microbeads and T-lymphocytes eliminated CD8 positivity within complexes (Figure 2D), suggesting a lack of interaction between microbeads and CD8 T-lymphocytes in the presence of MDSCs.
Figure 2. MDSCs sequester CD3/28 microbeads and prevent their contact with T-lymphocytes.
A, 4x photomicrographs of round-bottom wells containing the indicated cell type(s) and/or CD3/28 microbeads after 24 hours in culture. All components were added to culture in equal ratios. B, 40x photomicrographs of CD3/28 microbeads cultured for 24 hours alone or in the presence of MDSCs at a 1:1 ratio. C&D, CD3/28 microbeads and T-lymphocytes with or without MDSCs as indicated were co-cultured in equal ratios for 3 days. Microbeads were then magnetically isolated, and microbead:cellular complexes were stained with fluorophore conjugates antibodies as indicated and analyzed via flow cytometry. Left panels, quantification of complexes positive for markers as indicated, right panels, representative dot plots or histograms. All results shown are representative of a least 3 independent assays. TL, T-lymphocytes.
3.3 MDSCs sequester CD3/28 microbeads through attempted phagocytosis
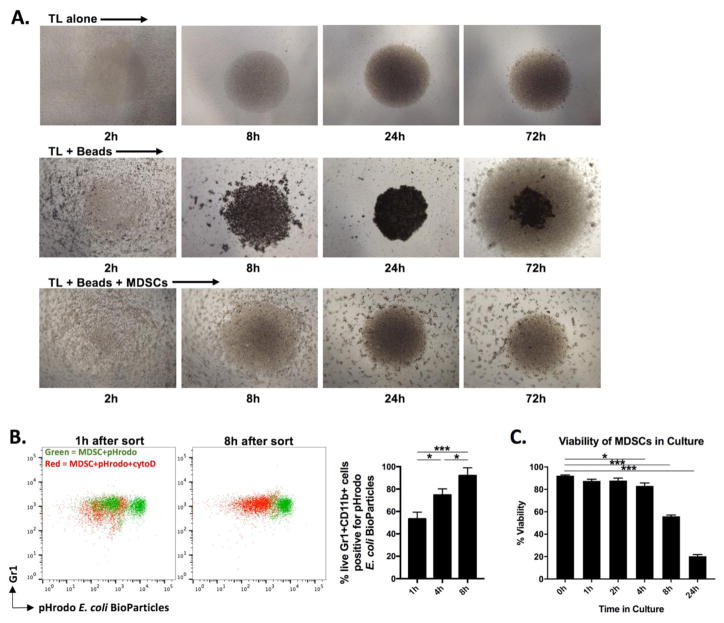
As a heterogeneous population of myeloid cells, MDSCs possess phagocytic potential. We hypothesized that MDSC sequestration of microbeads was caused by attempted phagocytosis. A time course of photomicrographs was performed to identify the time required in co-culture for MDSCs to sequester microbeads away from the T-lymphocytes. By 24 hours, in the absence of MDSCs, the microbeads appear settled adjacent to the T-lymphocytes in the base of the well (Figure 3A, middle row). Robust expansion of T-lymphocytes around the pooled microbeads is demonstrated at 72h. However, in the presence of MDSCs, microbeads remain clearly scattered at 24- and 72-hour time points (Figure 3A, bottom row). This suggests that MDSC-mediated sequestration of microbeads is occurring within the first 24 hour of culture. To evaluate the phagocytic potential of MDSCs after sorting, cells were exposed to E. coli BioParticles that become fluorescent upon entry into the low pH microenvironment of a phagosome. Time course evaluation of MDSC phagocytic capacity revealed an increase with time up 8 hours (Figure 3B). Use of cytochalasin D, an inhibitor of phagocytosis, revealed that MDSC positivity for BioParticles was specifically due to phagocytosis and not due to non-specific staining of non-viable cells. Evaluation of phagocytic activity in MDSCs beyond the 8-hour time point was impractical due to loss of cell viability. To assess MDSC viability over time in culture after sorting, we assess exclusion of PI. While viability is maintained for the first 8 hours, MDSCs subsequently rapidly loose viability and most are PI positive within 24 hours (Figure 3C). Poor survival of MDSCs in culture without supplemented cytokines has been described [7]. Cumulatively, these data indicate that sorted MDSC in culture acquire increasing phagocytic capacity within 8 hours, but then rapidly loose viability. This acquired phagocytic activity correlates with their ability to sequester microbeads away from T-lymphocytes within the first 24 hours of culture.
Figure 3. MDSCs sequester microbeads through attempted phagocytosis.
A, 4x Photomicrographs of T-lymphocytes with or without CD3/28 microbeads and MDSCs as indicated at various time points after plating. B, MDSCs were sorted and incubated with pHrodo E. coli BioParticles at 100 μg/1×105 cells for 90 minutes, then stained with cell surface antibodies and analyzed with flow cytometry. Representative dot plots (left panels) are quantified (right panel) at 1, 4 and 8 hours after sorting. Green dots represent MDSC exposed to pHrodo alone, red dots represent MDSCs exposed to pHrodo in the presence of 10 μM cytochalasin D (inhibitor of phagocytosis). These stains were performed in separate tubes and shown are representative overlay dot plots demonstrating loss of BioParticle positivity in 7AAD-CD11b+ cells the presence of cytochalasin D. C, Sorted MDSCs were cultured in complete media without supplemental cytokines, stained with acridine orange and propidium iodide, and positivity was quantified on a Cellometer Auto 2000 fluorescent imager at indicated time points. *, p<0.05; **, p<0.01; ***, p<0.001; ANOVA. TL, T-lymphocytes.
3.4 Microbead sequestration artifact is avoided in an MDSC suppression assay through the use of plate-bound CD3/28 antibodies
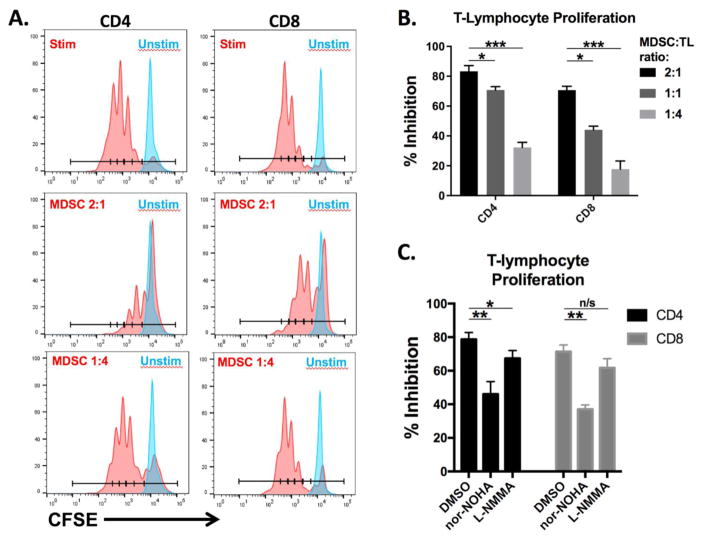
In order to remove the potential for microbead sequestration by MDSCs, we next evaluated the immunosuppressive potential of MOC1-tumor bearing mouse peripheral MDSCs in an assay using plate-bound anti-CD3/28 stimulation of T-lymphocyte proliferation as previously described [12]. Significant, dilution-dependent suppression (p<0.001 for all dilutions tested) of T-lymphocyte proliferation was observed (Figure 4A, quantified in 4B). In contrast to MDSC suppression of T-lymphocyte proliferation in the presence of CD3/28 microbeads, arginase (nor-NOHA) and to some degree iNOS (L-NMMA) inhibitors were able to partially reverse this suppression, consistent with mechanisms of MDSC suppression established in many solid tumor models [12–17]. Reversal of MDSC suppression with arginase and iNOS inhibition was incomplete, consistent with multiple known mechanisms utilized by MDSC to suppress T-lymphocyte function [1, 20].
Figure 4. MDSCs suppress plate-bound CD3/28-stimulated T-lymphocyte proliferation.
Sorted MDSCs were co-cultured with CFSE-labeled T-cells for 4 hours, then stimulated with plate-bound anti-CD3/28 for 3 days. A, Flow cytometry histograms demonstrating the CFSE distribution of T-cells incubated with MDSCs at the indicated MDSC:T-lymphocyte ratios on CD3/28 coated plates. B, quantification of these data. C, Sorted MDSCs and CFSE-labelled T-lymphocytes were incubated for 4 hours with 300 μM arginase (nor-NOHA) or iNOS (L-NMMA) inhibitors or an equivalent concentration of DMSO before being added to plates coated with CD3/28. Results shown are representative of at least three independent assays with similar trends. *, p<0.05; **, p<0.01; ***, p<0.001; ANOVA. Nor-NOHA, arginase inhibitor; L-NMMA, iNOS inhibitor; TL, T-lymphocytes.
5. Discussion
These data demonstrate artefactual immunosuppression observed by the addition of MDSCs from our MOC syngeneic model to CD3/28 microbead-stimulated T-lymphocytes. When applied in our model, this microbead-based assay appears to evaluate the ability of MDSCs to phagocytize microbeads, rather than the true ability of MDSCs to suppress T-lymphocyte proliferation through physiologic mechanisms such as arginine depletion from the media and production of nitric oxide. Other mechanisms of MDSC suppression have been described within model suppression systems, including loss of CD3 antibody integrity [20]. This likely accounts for why reversal of MDSC suppression was incomplete with use of arginine and iNOS inhibitors. However, all physiologic mechanisms of MDSC suppression would be masked and irreversible in our system due to bead sequestration away from T-lymphocytes.
Others have raised concerns about the validity of microbead-based MDSC suppression assays, hypothesizing that artifact could be caused by MDSCs altering stimulatory antibodies, or attempting to phagocytize stimulatory microbeads [8]. Our results provide convincing evidence that, at least in our model system, the use of CD3/28 microbeads leads to artefactual suppression of T-cell proliferation. We show both qualitatively through photomicrographs and quantitatively through flow cytometry that MDSCs in culture bind to anti-CD3/28 microbeads, ultimately sequestering them from and preventing their contact with T-lymphocytes. By demonstrating the ability of MDSCs from our model to phagocytize fluorescent BioParticles within an 8-hour timeframe of preserved viability, we provide a mechanistic explanation for this MDSC:microbead interaction.
The poor viability of myeloid cells in culture has been well described, and our data further confirms these findings. Youn, et al. also observed MDSC death within 24 hours of culture in the absence of growth factors, with increased 24-hour viability to 60% in the presence of GM-CSF [7]. However, even in the presence of GM-CSF, MDSCs differentiated into neutrophils within 24 hours and traded immunosuppressive for phagocytic capacity. In contrast to this description of MDSCs as poorly phagocytic upon immediate isolation with the subsequent acquisition of phagocytic activity when cultured in GM-CSF, we observed some degree of phagocytic activity of our MDSCs nearly immediately upon isolation. It is possible that the MDSCs from our oral cavity cancer model are more phagocytic than those derived from the EL4 model, or that our MDSCs differentiate into phagocytes more rapidly in culture. Regardless, this variation in phagocytic activity from one model to another supports the necessity for model-specific validation of microbead-based MDSC suppression assays. This could be accomplished through the inclusion of photomicrographs alongside MDSC suppression data from microbead-based assays in order to demonstrate a lack of bead scattering artifact. Alternatively, phagocytosis data could be included to verify the model-specific lack of MDSC phagocytic activity prior to evaluating their immunosuppressive function in a microbead-based assay. More simply, utilizing plate-bound CD3/28 antibodies for T-lymphocyte stimulation should avoid the potential for phagocytosis artifact in assays designed to evaluate the immunosuppressive effect of myeloid cells that may have phagocytic capacity. The use of pre-activated T-lymphocytes that no longer are dependent upon the continued presence of CD3/28 antibody stimuli [20] would be another alternative method to avoid bead-related artifact. Antibody coated microbeads certainly likely retain their utility in assays designed to evaluate the immunosuppressive effect of non-phagocytic cells, such as regulatory T-lymphocytes.
An additional concern in assays utilizing antibodies as T-lymphocyte stimuli involves the effect that reactive oxygen species (ROS) may have on the ability of antibodies to bind specific antigen. MDSC typically generate ROS [5,7,18] that have the capacity to alter how CD3/28 antibodies activate T-lymphocytes [19,20]. While this is considered a mechanism of MDSC T-lymphocyte suppression itself, it potentially masks the effects of arginine depletion and nitric oxide production by MDSCs in a suppression assay. If desired, this direct effect could be avoided by using antigen-specific cellular stimuli, such as antigen-specific T-lymphocytes stimulated with antigen pulsed presenting cells. In our experience, the T-lymphocyte stimulus in antigen-specific systems is robust and often requires higher MDSC:T-lymphocyte ratios to demonstrate suppressive effect (unpublished data).
In conclusion, we have identified artefactual suppression of T-lymphocyte proliferation in a microbead-based MDSC suppression assay due to microbead sequestration, and provide evidence that reversible MDSC suppression can be achieved by eliminating the microbeads in an assay utilizing plate bound stimulatory antibodies. While CD3/28 coated microbeads are a useful tool in the immunology laboratory, we caution investigators to be critical of their use in assays where sequestration of microbeads away from targets cells by myeloid cells with phagocytic capacity could lead to artefactual results. In these situations, model-dependent validation of the lack of myeloid sequestration of microbeads or simply using alternative methods of T-lymphocyte stimulation that avoid the use of microbeads are warranted.
Highlights.
In our syngeneic carcinogen-induced model of oral cavity cancer, sorted myeloid derived suppressor cells (MDSCs) rapidly gained phagocytic capacity before losing viability in culture
This led to sequestration of CD3/28 coated microbeads away from target T-lymphocytes in a suppression assay and resulted in artefactual suppression of T-lymphocyte proliferation that was not reversible with inhibitors of arginase and iNOS
Eliminating microbeads from the assay and utilizing plate bound CD3/28 antibodies allowed assessment of physiologic T-lymphocyte suppression by MDSCs that was partially reversible with inhibitors of arginase and iNOS
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders intramural project number DC DC000087-01 and by a Johns Hopkins Specialized Program of Research Excellence (SPORE) Head and Neck Cancer Pilot Award. We thank Drs. Jim Hodge, Nicole Schmitt and Zhong Chen for their critical reviews of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1:95–103. [PubMed] [Google Scholar]
- 3.Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:39–48. doi: 10.1158/1078-0432.CCR-14-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–30. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youn J-I, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91:167–81. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hock BD, McKenzie JL. Suppression of CD3/CD28 antibody stimulated responses by human granulocytic myeloid-derived suppressor cells: fact or artefact? Immunol Lett. 2013;152:151–2. doi: 10.1016/j.imlet.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Judd NP, Winkler AE, Murillo-Sauca O, Brotman JJ, Law JH, Lewis JS, et al. ERK1/2 regulation of CD44 modulates oral cancer aggressiveness. Cancer Res. 2012;72:365–74. doi: 10.1158/0008-5472.CAN-11-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cash H, Shah S, Moore E, Caruso A, Uppaluri R, Van Waes C, et al. mTOR and MEK1/2 inhibition differentially modulate tumor growth and the immune microenvironment in syngeneic models of oral cavity cancer. Oncotarget. 2015;6:36400–17. doi: 10.18632/oncotarget.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quah BJC, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–56. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- 12.Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 2013;123:1580–9. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra67. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beury DW, Carter KA, Nelson C, Sinha P, Hanson E, Nyandjo M, et al. Myeloid-Derived Suppressor Cell Survival and Function Are Regulated by the Transcription Factor Nrf2. J Immunol. 2016;196:3470–8. doi: 10.4049/jimmunol.1501785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filatenkov A, Baker J, Mueller AMS, Kenkel J, Ahn G-O, Dutt S, et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res. 2015;21:3727–39. doi: 10.1158/1078-0432.CCR-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–45. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Wang L, Fan J, Ye C, Dominguez D, Zhang Y, et al. Host miR155 promotes tumor growth through a myeloid-derived suppressor cell-dependent mechanism. Cancer Res. 2015;75:519–31. doi: 10.1158/0008-5472.CAN-14-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimitrov JD, Vassilev TL, Andre S, Kaveri SV, Lacroix-Desmazes S. Functional variability of antibodies upon oxidative processes. Autoimmun Rev. 2008;7:574–8. doi: 10.1016/j.autrev.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Hock BD, Taylor KG, Cross NB, Kettle AJ, Hampton MB, McKenzie JL. Effect of activated human polymorphonuclear leucocytes on T lymphocyte proliferation and viability. Immunology. 2012;137:249–58. doi: 10.1111/imm.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]