Figure 1.
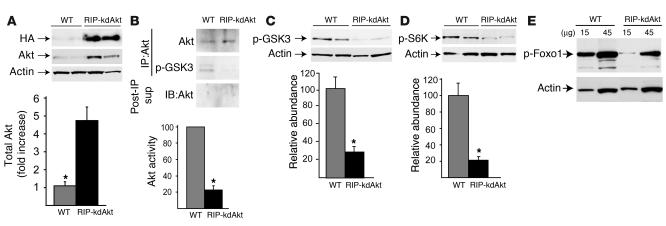
Overexpression of kdAkt inhibits Akt activity and phosphorylation of Akt targets in RIP-kdAkt islets. (A) Total pancreatic islet lysates from RIP-kdAkt and WT mice were immunoblotted with Ab’s against HA, Akt, or actin. Semiquantitation of total Akt protein level in islets from RIP-kdAkt and WT mice was adjusted to actin as loading control (n = 6). (B) Total in vitro Akt kinase activity from 400 μg of islet lysates. Upper blot: Immunoblotting for Akt in the immunoprecipitate. Middle blot and bar graph: In vitro Akt kinase activity assayed by immunoblotting with anti_phospho-GSK3 Ab’s (p-GSK3) with semiquantitative analysis (n = 3). Lower blot: Immunoblotting for Akt in the post-immunoprecipitation supernatant (Post-IP sup). (C and D) Phosphorylation status in islet lysates and semiquantitative analysis of band intensities for phospho-GSK3 (Ser9; n = 5) (C) and phospho-S6K (Thr389; n = 6) (D) in transgenics and WT mice. (E) Immunoblotting for phospho-Foxo1 (Ser256) and actin in lysates from RIP-kdAkt and WT islets. The data are representative of 3 independent experiments done in duplicate. Data for phospho-GSK3 and phospho-S6K were adjusted to actin and are presented as mean ± SE. *P < 0.05.